
£Ø17·Ö£©
£Ø1£©ÓŅĶ¼ĖłŹ¾ŹĒŹµŃéŹŅÖŠÖĘČ”ĘųĢåµÄŅ»ÖÖ¼ņŅ××°ÖĆ”£
¢ŁĒė¼ņŹö¼ģŃéĖüµÄĘųĆÜŠŌµÄ·½·Ø ”£
¢ŚĄūÓĆÓŅĶ¼ĖłŹ¾×°ÖĆæÉŅŌÖĘČ” ĘųĢå£ØĢī·“Ó¦Īļדæö¼°·¢Éś·“Ó¦ŹĒ·ńŠčŅŖµÄĢõ¼ž£©”£
£Ø2£©ÕÅĆ÷Ķ¬Ń§Éč¼ĘĻĀĶ¼ĖłŹ¾×°ÖĆ£¬ÓĆ“ÖĢśĮ£Óė189g”¤L-1ĻõĖį·“Ó¦ÖĘČ”NOĘųĢ唣Ēė»Ų“šÓŠ¹ŲĪŹĢā”£
¢Ł189g”¤L-1ĻõĖįµÄĪļÖŹµÄĮæÅضČĪŖ ”£
¢ŚNOĘųĢåÄÜÓĆÅÅĖ®·ØŹÕ¼Æ£¬¶ų²»ÄÜÓĆÅÅæÕĘų·ØŹÕ¼ÆµÄæÉÄÜŌŅņŹĒ ”£
¢Ūµ±ÕŅÄ©Ö¹Ė®¼Ša£¬¹Ų±ÕÖ¹Ė®¼ŠbŹ±£¬A×°ÖƵÄøÉŌļ¹ÜÖŠ¹Ū²ģµ½µÄĻÖĻóŹĒ ”£B×°ÖĆÉÕ±ÖŠŅŗĢåµÄ×÷ÓĆŹĒ ”£µ±A×°ÖĆÖŠĘųĢå¼øŗõĪŽÉ«Ź±£¬“ņæŖÖ¹Ė®¼Šb£¬¹Ų±ÕÖ¹Ė®¼Ša£¬æÉÓĆC×°ÖĆŹÕ¼ÆNOĘųĢ唣
¢ÜøĆŹµŃéÖŠ£¬ČōČ„µōB×°ÖĆ£¬¶ŌŹµŃé½į¹ūŹĒ·ńÓŠÓ°Ļģ£æ ”£
¢Ż½«a molĢśÓėŗ¬b molHNO3µÄĻ”ČÜŅŗ³ä·Ö·“Ó¦ŗó£¬ČōHNO3µÄ»¹Ō²śĪļÖ»ÓŠNO£¬·“Ó¦ŗóFeŗĶHNO3¾łĪŽŹ£Óą£¬Ōņ·“Ó¦ÖŠ×ŖŅʵē×ÓµÄĪļÖŹµÄĮæŹĒ £ØÓĆŗ¬aŗĶbµÄ“śŹżŹ½±ķŹ¾£©”£
¢ŽĶźČ«·“Ó¦ŗó£¬A×°ÖĆÉÕ±Ąļŗ¬ĢśµÄ¼ŪĢ¬æÉÄÜŹĒ ”£ĻÖÓŠŅĒĘ÷ŗĶŅ©Ę·£ŗŹŌ¹ÜŗĶ½ŗĶ·µĪ¹Ü”£0.1mol”¤L-1KSCNČÜŅŗ”¢0.1mol”¤L-1KIČÜŅŗ”¢0.2mol”¤L-1ĖįŠŌKMnO4ČÜŅŗ”¢ĀČĖ®µČ”£ĒėÄćÉč¼ĘŅ»øö¼ņµ„ŹµŃ飬Ģ½¾æÉĻŹöÅŠ¶Ļ£¬ĢīŠ“ĻĀĮŠŹµŃé±Øøę£ŗ
| ŹµŃé²½Öč | ²Ł×÷ | ĻÖĻóÓė½įĀŪ |
| µŚŅ»²½ | ȔɣĮæŅŗĢå×°ÓŚŹŌ¹Ü£¬ĻņŹŌ¹ÜÖŠµĪČė¼øµĪKSCNČÜŅŗ |
|
| µŚ¶ž²½ |
| ČōČÜŅŗ×ĻÉ«ĶĖČ„£¬ŌņĖµĆ÷ŗ¬ÓŠFe2+£¬ČōĪŽĆ÷ĻŌ±ä»Æ£¬ŌņĖµĆ÷²»ŗ¬Fe2+ |
£Ø1£©¹Ų±ÕÖ¹Ė®¼ŠB£¬“ÓA“¦¼ÓČėĖ®£¬Ź¹UŠĪ¹ÜĮ½¶ĖŠĪ³ÉŅŗĆę²ī£¬Ņ»¶ĪŹ±¼äŗó£¬ŅŗĆę²īƻӊ±ä»Æ£¬ĖµĆ÷ĘųĆÜŠŌĮ¼ŗĆ£Ø2·Ö”£Ć»ÓŠ¹Ų±ÕÖ¹Ė®¼ŠB²»µĆ·Ö£©
¢Śæéד»ņ½Ļ“óæÅĮ£×“¹ĢĢåÓėŅŗĢå²»ŠčŅŖ¼ÓČČ·“Ӧɜ-³ÉµÄ£Ø2·Ö”£Ö»“š¹ĢĢåæŪ1·Ö£¬²»ĖµĆ÷Ģõ¼žæŪ1·Ö£©
£Ø2£©¢Ł3mol”¤L-1£Ø2·Ö”£Ā©µ„Ī»æŪ1·Ö£©
¢ŚNOÓėæÕĘųÖŠµÄO2·“Ó¦£»NOÓėæÕĘųĆÜ¶Č½Ó½ü£Ø2·Ö£©
¢ŪÓŠŗģ×ŲÉ«ĘųĢå³öĻÖ£Ø1·Ö£©ĪüŹÕNO2£Ø1·Ö£©
¢ÜĪŽÓ°Ļģ£Ø1·Ö£©
¢Ż![]() mol£Ø2a mol»ņ3amol»ņ2amol~3amol£©
mol£Ø2a mol»ņ3amol»ņ2amol~3amol£©
£Ø2·Ö”£ÓĆŗ¬aµÄ“śŹżŹ½±ķ“ļŹ±£¬“š²»Č«µĆ1·Ö£©
¢Ž +2¼Ū»ņ+3¼Ū»ņ+2¼ŪŗĶ+3¼Ū£Ø2·Ö”£“š²»Č«µĆ1·Ö£©
ČōČÜŅŗ±äŃŖŗģÉ«£¬ĖµĆ÷ŗ¬Fe3+£¬ČōČÜŅŗĪŽĆ÷ĻŌ±ä»Æ£¬ĖµĆ÷²»ŗ¬Fe3+£Ø1·Ö£©
ȔɣĮæŅŗĢå×°ÓŚŹŌ¹Ü£¬ĻņŹŌ¹Ü-ÖŠµĪČė¼øµĪĖįŠŌKMnO4ČÜŅŗ£Ø1·Ö£©
½āĪö:



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
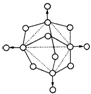 Į×ŌŚæÕĘųÖŠ³ä·ÖČ¼ÉÕ£¬Éś³ÉXµÄ·Ö×Ó½į¹¹ČēÓŅĶ¼ĖłŹ¾£®Ķ¼ÖŠŹµĻß±ķŹ¾»Æѧ¼ü£¬Ō²Č¦±ķŹ¾Ō×ӣز»æ¼ĀĒŌ×ÓĢå»ż“󊔣©
Į×ŌŚæÕĘųÖŠ³ä·ÖČ¼ÉÕ£¬Éś³ÉXµÄ·Ö×Ó½į¹¹ČēÓŅĶ¼ĖłŹ¾£®Ķ¼ÖŠŹµĻß±ķŹ¾»Æѧ¼ü£¬Ō²Č¦±ķŹ¾Ō×ӣز»æ¼ĀĒŌ×ÓĢå»ż“󊔣©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½Ī÷“óѧø½Źō֊ѧø߶žŹī¼Ł8ŌĀæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
(10·Ö)ŹµĮ³ŹŅ²ÉÓĆMgCl2”¢AlCl3µÄ»ģŗĻČÜŅŗÓė¹żĮæ°±Ė®·“Ó¦ÖʱøMgAl2O4¶žÖ÷ŅŖĮ÷³ĢČēĻĀ: 
(1)ĪŖŹ¹Mg2£«”¢Al3£«Ķ¬Ź±Éś³É³Įµķ£¬Ó¦ĻČĻņ³Įµķ·“Ó¦Ę÷ÖŠ¼ÓČė (Ģī”°A”±»ņ”°B")£¬ŌŁµĪ¼ÓĮķŅ»·“Ó¦Īļ”£
(2)ČēÓŅĶ¼ĖłŹ¾£¬¹żĀĖ²Ł×÷ÖŠµÄŅ»“¦“ķĪóŹĒ ”£
(3)Ļ“µÓ³ĮµķµÄ·½·ØŹĒ £»ÅŠ¶ĻĮ÷³ĢÖŠ³ĮµķŹĒ·ńĻ“¾»ĖłÓƵďŌ¼ĮŹĒ ”£øßĪĀ±ŗÉÕŹ±£¬ÓĆÓŚŹ¢·Å¹ĢĢåµÄŅĒĘ÷Ćū³ĘŹĒ ”£
(4)ĪŽĖ®AlCl3(183”ęÉż»Ŗ)Óö³±ŹŖæÕĘų¼“²śÉś“óĮæ°×Īķ£¬ŹµŃéŹŅæÉÓĆĻĀĮŠ×°ÖĆÖʱø”£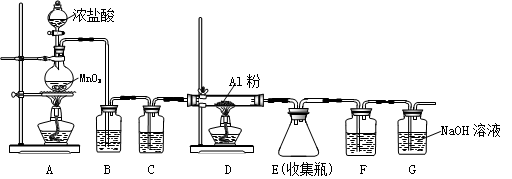
Š“³öAÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£×°ÖĆBÖŠŹ¢·Å±„ŗĶNaClČÜŅŗ,øĆ×°ÖƵÄÖ÷ŅŖ×÷ÓĆŹĒ ”£FÖŠŹŌ¼ĮµÄ×÷ÓĆŹĒ ”£ÓĆŅ»¼žŅĒĘ÷×°ĢīŹŹµ±ŹŌ¼ĮŗóŅ²æÉĘšµ½FŗĶGµÄ×÷ÓĆ£¬Ėł×°ĢīµÄŹŌ¼ĮĪŖ _”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğÕć½Ź”ĮłŠ££ØŹ”Ņ»¼¶ÖŲµćŠ££©øßČż3ŌĀĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
æŖ·¢ŠĀÄÜŌ“ŗĶČż·Ļ“¦Ąķ¶¼ŹĒæɳ֊ų·¢Õ¹µÄÖŲŅŖ·½Ćę”£
£Ø1£©ÓÉĢ¼µÄŃõ»ÆĪļÖ±½ÓŗĻ³ÉŅŅ“¼Č¼ĮĻŅŃ½ųČė“ó¹ęÄ£Éś²ś”£Čē²ÉČ”ŅŌCOŗĶH2ĪŖŌĮĻŗĻ³ÉŅŅ“¼£¬»Æѧ·“Ó¦·½³ĢŹ½£ŗ2CO(g)+4H2(g) CH3CH2OH(g)+H2O(g)£»ČōĆܱÕČŻĘ÷ÖŠ³äÓŠ10 mol COÓė20mol H2£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³ÉŅŅ“¼£ŗCOµÄ×Ŗ»ÆĀŹ(¦Į)ÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£
CH3CH2OH(g)+H2O(g)£»ČōĆܱÕČŻĘ÷ÖŠ³äÓŠ10 mol COÓė20mol H2£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³ÉŅŅ“¼£ŗCOµÄ×Ŗ»ÆĀŹ(¦Į)ÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£

¢ŁČōA”¢BĮ½µć±ķŹ¾ŌŚÄ³Ź±æĢ“ļµ½µÄĘ½ŗāדĢ¬£¬“ĖŹ±ŌŚAµćŹ±ČŻĘ÷µÄĢå»żĪŖ10L£¬ŌņøĆĪĀ¶ČĻĀµÄĘ½ŗā³£Źż£ŗK£½??????? £»
¢ŚČōA”¢CĮ½µć¶¼±ķŹ¾“ļµ½µÄĘ½ŗāדĢ¬£¬Ōņ×Ō·“Ó¦æŖŹ¼µ½“ļĘ½ŗāדĢ¬ĖłŠčµÄŹ±¼ätA???? tC£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©”£
¢Ū¹¤ŅµÉĻ»¹æÉŅŌ²ÉČ”ŅŌCO2ŗĶH2ĪŖŌĮĻŗĻ³ÉŅŅ“¼£¬²¢ĒŅøü±»»Æѧ¹¤×÷ÕßĶĘ³ē£¬µ«ŹĒŌŚĻąĶ¬Ģõ¼žĻĀ£¬ÓÉCOÖĘČ”CH3CH2OHµÄĘ½ŗā³£ŹżŌ¶Ō¶“óÓŚÓÉCO2ÖĘČ”CH3CH2OH µÄĘ½ŗā³£Źż”£ĒėĶĘ²ā»Æѧ¹¤×÷ÕßČĻæÉÓÉCO2ÖĘČ”CH3CH2OHµÄÓŵćÖ÷ŅŖŹĒ£ŗ???????????????????????????????????? ”£
£Ø2£©ÄæĒ°¹¤ŅµÉĻŅ²æÉŅŌÓĆCO2Ą“Éś²ś¼×“¼”£Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦CO2(g)£«3H2(g) CH3OH(g)£«H2O(g)”£Čō½«6mol CO2ŗĶ8 mol H2³äČė2LµÄĆܱÕČŻĘ÷ÖŠ£¬²āµĆH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆµÄĒśĻßČēÓŅĶ¼ĖłŹ¾£ØŹµĻߣ©”£
CH3OH(g)£«H2O(g)”£Čō½«6mol CO2ŗĶ8 mol H2³äČė2LµÄĆܱÕČŻĘ÷ÖŠ£¬²āµĆH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆµÄĒśĻßČēÓŅĶ¼ĖłŹ¾£ØŹµĻߣ©”£

¢ŁĒėŌŚ“šĢā¾ķĶ¼ÖŠ»ę³ö¼×“¼µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆĒśĻß”£
¢Ś½öøıäijŅ»ŹµŃéĢõ¼žŌŁ½ųŠŠĮ½“ĪŹµŃ飬²āµĆH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆČēĶ¼ÖŠŠéĻßĖłŹ¾£¬ĒśĻßI¶ŌÓ¦µÄŹµŃéĢõ¼žøıäŹĒ????????? ? £¬ĒśĻߢņ¶ŌÓ¦µÄŹµŃéĢõ¼žøıäŹĒ??????? ”£
£Ø3£©HgŹĒĖ®ĢåĪŪČ¾µÄÖŲ½šŹōŌŖĖŲÖ®Ņ»”£Ė®ČÜŅŗÖŠ¶ž¼Ū¹ÆµÄÖ÷ŅŖ“ęŌŚŠĪĢ¬ÓėCl”„”¢OH”„µÄÅØ¶Č¹ŲĻµČēÓŅĶ¼ĖłŹ¾£ŪĶ¼ÖŠµÄĪļÖŹ»ņĮ£×ÓÖ»ÓŠHg(OH)2ĪŖÄŃČÜĪļ£»pCl=£1gc(Cl”„)£Ż

¢ŁĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ????? ”£
A£®ĪŖĮĖ·ĄÖ¹Hg2£«Ė®½ā£¬ÅäÖĘHg(NO3)2ČÜŅŗŹ±Ó¦½«Hg(NO3)2¹ĢĢåČÜÓŚÅØĻõĖįŗóŌŁĻ”ŹĶ
B£®µ±c(C1”„) £½10”„1 mol”¤L”„1Ź±£¬¹ÆŌŖĖŲŅ»¶ØČ«²æŅŌHgCl42”„ŠĪŹ½“ęŌŚ
C£®HgCl2ŹĒŅ»ÖÖČõµē½āÖŹ£¬ĘäµēĄė·½³ĢŹ½ŹĒ£ŗHgCl2£½HgCl£« + C1”„
D£®µ±ČÜŅŗpH±£³ÖŌŚ4£¬pClÓÉ2øıäÖĮ6Ź±£¬æÉŹ¹HgCl2×Ŗ»ÆĪŖHg(OH)2
¢ŚHgCl2ÓÖ³Ę”°Éż¹Æ”±£¬ČŪµć549K£¬¼ÓČČÄÜÉż»Ŗ£¬Ę侧ĢåŹĒ????? £ØĢī¾§ĢåĄąŠĶ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ½ĖÕŹ”Ęō¶«ŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©¹Ģ¶ØŗĶĄūÓĆCO2ÄÜÓŠŠ§µŲĄūÓĆ׏Ō“£¬²¢¼õÉŁæÕĘųÖŠµÄĪĀŹŅĘųĢ唣¹¤ŅµÉĻÓŠŅ»ÖÖÓĆCO2Ą“Éś²ś¼×“¼Č¼ĮĻµÄ·½·Ø£ŗCO2(g)£«3H2(g) CH3OH(g)£«H2O(g)”÷H£½£49£®0kJ”¤mol£1”£Ä³æĘѧŹµŃ齫6molCO2ŗĶ8molH2³äČė2LĆܱÕČŻĘ÷ÖŠ£¬²āµĆH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆČēÓŅĶ¼ĖłŹ¾£ØŹµĻߣ©”£Ķ¼ÖŠŹż¾Ża£Ø1£¬6£©“ś±ķµÄŅāĖ¼ŹĒ£ŗŌŚl minŹ±H2µÄĪļÖŹµÄĮæŹĒ6mol”£
CH3OH(g)£«H2O(g)”÷H£½£49£®0kJ”¤mol£1”£Ä³æĘѧŹµŃ齫6molCO2ŗĶ8molH2³äČė2LĆܱÕČŻĘ÷ÖŠ£¬²āµĆH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆČēÓŅĶ¼ĖłŹ¾£ØŹµĻߣ©”£Ķ¼ÖŠŹż¾Ża£Ø1£¬6£©“ś±ķµÄŅāĖ¼ŹĒ£ŗŌŚl minŹ±H2µÄĪļÖŹµÄĮæŹĒ6mol”£
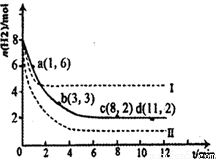
¢ŁĻĀĮŠŹ±¼ä¶ĪĘ½¾ł·“Ó¦ĖŁĀŹ×ī“óµÄŹĒ__________£¬×īŠ”µÄŹĒ______________”£
A£®0”«1min B£®1”«3min C£®3”«8min D£®8”«11min
¢Ś½öøıäijŅ»ŹµŃéĢõ¼žŌŁ½ųŠŠĮ½“ĪŹµŃé²āµĆH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆČēĶ¼ÖŠŠéĻßĖłŹ¾£¬ĒśĻߢń¶ŌÓ¦µÄŹµŃéøıäµÄĢõ¼žŹĒ________£¬ĒśĻߢņ¶ŌÓ¦µÄŹµŃéøıäµÄĢõ¼žŹĒ_________”£
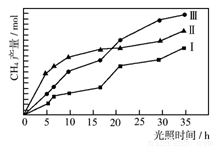
£Ø2£©ĄūÓĆ¹āÄÜŗĶ¹ā“߻ƼĮ£¬æɽ«CO2ŗĶH2O(g)×Ŗ»ÆĪŖCH4ŗĶO2”£×ĻĶā¹āÕÕÉ䏱£¬µČĮæµÄCO2ŗĶH2O(g)ŌŚ²»Ķ¬“߻ƼĮ(¢ń”¢¢ņ”¢¢ó)×÷ÓĆĻĀ£¬CH4²śĮæĖę¹āÕÕŹ±¼äµÄ±ä»ÆČēĶ¼ĖłŹ¾”£ŌŚ0~30 hÄŚ£¬CH4µÄĘ½¾łÉś³ÉĖŁĀŹv(¢ń)”¢v(¢ņ)ŗĶv(¢ó)“ӓ󵽊”µÄĖ³ŠņĪŖ ”£·“Ó¦æŖŹ¼ŗóµÄ12Š”Ź±ÄŚ£¬ŌŚµŚ___________Ö֓߻ƼĮµÄ×÷ÓĆĻĀ£¬ŹÕ¼ÆµÄCH4×ī¶ą”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģÕć½Ź”øßŅ»ĻĀѧʌʌ֊¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø8·Ö£©ÓĆ“æ¾»µÄæéדµÄĢ¼ĖįøĘÓėĻ”ŃĪĖį·“Ó¦ÖĘČ”¶žŃõ»ÆĢ¼ĘųĢ壬Ēė»Ų“š£ŗ

£Ø1£©ŹµŃé¹ż³ĢČēÓŅĶ¼ĖłŹ¾£¬·ÖĪöÅŠ¶Ļ£ŗ______¶Ī»Æѧ·“Ó¦ĖŁĀŹ×īæģ£¬_______¶ĪŹÕ¼ÆµÄ¶žŃõ»ÆĢ¼ĘųĢå×ī¶ą”£
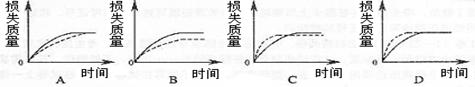
(2)ÉĻŹö·“Ó¦ÖŠ,A gæéדĢ¼ĖįøĘøś×ćĮæŃĪĖį·“Ó¦£¬·“Ó¦ĪļĻūŗĵÄÖŹĮæĖꏱ¼äµÄ±ä»ÆĒśĻßÓÉŹµĻß±ķŹ¾£¬ŌŚĻąĶ¬µÄĢõ¼žĻĀ£¬½«B g £ØA>B£©·ŪĩדĢ¼ĖįøĘÓė×ćĮæµÄĶ¬ÅضČŃĪĖį·“Ó¦£¬·“Ó¦ĪļĻūŗĵÄÖŹĮæĖꏱ¼äµÄ±ä»ÆĒśĻßÓÉŠéĻß±ķŹ¾”£Ōņ×ī·ūŗĻŹµ¼ŹĒéæöµÄĶ¼ĻńŹĒ________
£Ø3£©ĪŖĮĖ¼õ»ŗÉĻŹö·“Ó¦µÄĖŁĀŹ£¬ÓūĻņČÜŅŗÖŠ¼ÓČėĻĀĮŠĪļÖŹ£¬ÄćČĻĪŖæÉŠŠµÄŹĒ_________.
A.ÕōĮóĖ® B.ĀČ»ÆÄĘ C.ĻõĖį¼ŲČÜŅŗ D.ÅØŃĪĖį E.½µĪĀ F¼ÓČėMnO2
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com