
³¬ĻøŃõ»ÆĀĮŹĒŅ»ÖÖÖŲŅŖµÄ¹¦ÄÜĢÕ“ÉŌĮĻ”£
£Ø1£©ŹµŃéŹŅ³£ŅŌNH4Al£ØSO4£©2ŗĶNH4HCO3ĪŖŌĮĻ£¬ŌŚŅ»¶ØĢõ¼žĻĀĻČ·“Ӧɜ³É³ĮµķNH4AlO£ØOH£©HCO3£¬øĆ³ĮµķøßĪĀ·Ö½ā¼“µĆ³¬ĻøAl2O3”£NH4AlO£ØOH£©HCO3ČČ·Ö½āµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ_________________________”£
£Ø2£©NH4Al£ØSO4£©2”¤12H2OµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ453”£ÓūÅäÖĘ100 mL pHĪŖ2”¢ÅضČŌ¼ĪŖ0.1 mol”¤L-1µÄNH4Al£ØSO4£©2ČÜŅŗ£¬ÅäÖĘ¹ż³ĢĪŖ£ŗ
¢ŁÓĆĶŠÅĢĢģĘ½³ĘĮæNH4Al£ØSO4£©2”¤12H2O¹ĢĢå______________________g£»
¢Ś½«ÉĻŹö¹ĢĢåÖĆÓŚÉÕ±ÖŠ£¬_________________________”£
£Ø3£©ŌŚ0.1 mol”¤L-1 NH4Al£ØSO4£©2ČÜŅŗÖŠ£¬ĀĮø÷ŠĪĢ¬µÄÅØ¶Č£ØŅŌAl3+¼Ę£©µÄ¶ŌŹż£Ølgc£©ĖęČÜŅŗpH±ä»ÆµÄ¹ŲĻµ¼ūĻĀĶ¼£ŗ
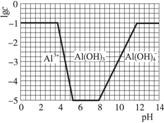
¢ŁÓĆNaOHČÜŅŗµ÷½Ś£Ø2£©ÖŠČÜŅŗpHÖĮ7£¬øĆ¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ÓŠ___________”£
¢ŚĒėŌŚ“šĢāæصÄæņĶ¼ÖŠ£¬»³ö0.01 mol”¤L-1 NH4Al£ØSO4£©2ČÜŅŗÖŠĀĮø÷ŠĪĢ¬µÄÅØ¶ČµÄ¶ŌŹżlgcĖęČÜŅŗpH±ä»ÆµÄ¹ŲĻµĶ¼£¬²¢½ųŠŠ±ŲŅŖ±ź×¢”£
”¾“š°ø”æ£Ø1£©2NH4AlO£ØOH£©HCO3 Al2O3+2CO2”ü+2NH3”ü+3H2O”ü
Al2O3+2CO2ӟ+2NH3ӟ+3H2Oӟ
£Ø2£©¢Ł4.5 ¢Ś¼ÓŹŹĮæĻ”ĮņĖįČܽā£¬ŌŁ¼ÓĖ®Ļ”ŹĶÖĮ100 mL
£Ø3£©¢ŁAl3++3OH-====Al(OH)3”ż H++OH-====H2O 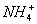 +OH-====NH3”¤H2O
+OH-====NH3”¤H2O
¢Ś
”¾½āĪö”æ(2)¢Łm£½0.1 mol”¤L-1”Į0.1 L”Į453 g”¤mol-1£½4.5 g
¢ŚŅņÅäÖĘČÜŅŗµÄpHĪŖ2£¬ĖłŅŌÅäÖĘČÜŅŗŹ±ŅŖ¼ÓČėŹŹĮæµÄĻ”ĮņĖį”£
£Ø3£©¢ŁpH£½7Ź±£¬Al£ØOH£©3²»Čܽā£¬Ö»ÓŠŅ»øöĄė×Ó·“Ó¦·¢Éś”£
¢Ś0.01 mol”¤L-1 NH4Al£ØSO4£©2ČÜŅŗµÄlgc£½-2”£


 ĆĻ½ØĘ½“ķĢā±¾ĻµĮŠ“š°ø
ĆĻ½ØĘ½“ķĢā±¾ĻµĮŠ“š°ø ³¬ÄÜѧµäÓ¦ÓĆĢāĢāæØĻµĮŠ“š°ø
³¬ÄÜѧµäÓ¦ÓĆĢāĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĻĀĮŠø÷×éĪļÖŹÖŠ£¬ŹōÓŚĶ¬ĻµĪļµÄŅ»×éŹĒ£Ø£©
A£® 1£¬1﹣¶žĀČŅŅĶéŗĶ1£¬2﹣¶žĀČŅŅĶé
B£® ±½ŗĶ¼×±½
C£® ŅŅ¶ž“¼ŗĶ±ūČż“¼
D£® ŅŅĻ©ŗĶ±ūČ²
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŌĮņĢśæó£ØÖ÷ŅŖ³É·ÖĪŖFeS2£©ĪŖŌĮĻÖʱøĀČ»ÆĢś¾§Ģå£ØFeCl3”¤6H2O£©µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
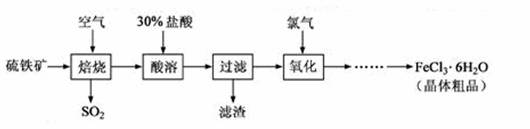
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬SO2×Ŗ»ÆĪŖSO3µÄ·“Ó¦ĪŖSO2£«O2 SO3£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK£½ £»¹żĮæµÄSO2ÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
SO3£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK£½ £»¹żĮæµÄSO2ÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ĖįČܼ°ŗóŠų¹ż³ĢÖŠ¾łŠč±£³ÖŃĪĖį¹żĮ棬ĘäÄæµÄŹĒ ”¢ ”£
£Ø3£©ĶØĀČĘųŃõ»ÆŹ±£¬·¢ÉśµÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»øĆ¹ż³Ģ²śÉśµÄĪ²ĘųæÉÓĆ¼īČÜŅŗĪüŹÕ£¬Ī²ĘųÖŠĪŪČ¾æÕĘųµÄĘųĢåĪŖ £ØŠ“»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
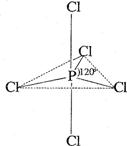 °×Į×£ØP4£©ŹĒĮ׵ĵ„ÖŹÖ®Ņ»£¬Ņ×Ńõ»Æ£¬ÓėĀ±ĖŲµ„ÖŹ·“Ӧɜ³ÉĀ±»ÆĮ×”£Ā±»ÆĮ×Ķس£ÓŠČżĀ±»ÆĮ×»ņĪåĀ±»ÆĮ×£¬ĪåĀ±»ÆĮ×·Ö×Ó½į¹¹£ØŅŌPCl5ĪŖĄż£©ČēÓŅĶ¼ĖłŹ¾”£øĆ½į¹¹ÖŠĀČŌ×ÓÓŠĮ½ÖÖ²»Ķ¬Ī»ÖĆ”£
°×Į×£ØP4£©ŹĒĮ׵ĵ„ÖŹÖ®Ņ»£¬Ņ×Ńõ»Æ£¬ÓėĀ±ĖŲµ„ÖŹ·“Ӧɜ³ÉĀ±»ÆĮ×”£Ā±»ÆĮ×Ķس£ÓŠČżĀ±»ÆĮ×»ņĪåĀ±»ÆĮ×£¬ĪåĀ±»ÆĮ×·Ö×Ó½į¹¹£ØŅŌPCl5ĪŖĄż£©ČēÓŅĶ¼ĖłŹ¾”£øĆ½į¹¹ÖŠĀČŌ×ÓÓŠĮ½ÖÖ²»Ķ¬Ī»ÖĆ”£
1£©6.20g°×Į×ŌŚ×ćĮæŃõĘųÖŠĶźČ«Č¼ÉÕÉś³ÉŃõ»ÆĪļ£¬·“Ó¦ĖłĻūŗĵÄŃõĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ L”£
ÉĻŹöČ¼ÉÕ²śĪļČÜÓŚĖ®Åä³É50.0mLĮ×Ėį£ØH3PO4£©ČÜŅŗ£¬øĆĮ×ĖįČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ mol”¤L-1”£
2£©ŗ¬0.300mol H3PO4µÄĖ®ČÜŅŗµĪ¼Óµ½ŗ¬0.500mol Ca(OH)2µÄŠüø”ŅŗÖŠ£¬·“Ó¦Ē”ŗĆĶźČ«£¬Éś³ÉlÖÖÄŃČÜŃĪŗĶ16.2g H2O”£øĆÄŃČÜŃĪµÄ»ÆѧŹ½æɱķŹ¾ĪŖ ”£
3£©°×Į×ŗĶĀČ”¢äå·“Ó¦£¬Éś³É»ģŗĻĀ±»ÆĮ×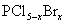 £Ø
£Ø £¬ĒŅxĪŖÕūŹż£©”£
£¬ĒŅxĪŖÕūŹż£©”£
Čē¹ūij»ģŗĻĀ±»ÆĮ×¹²ÓŠ3ÖÖ²»Ķ¬½į¹¹£Ø·Ö×ÓÖŠäåŌ×ÓĪ»ÖĆ²»ĶźČ«ĻąĶ¬µÄ½į¹¹£©£¬øĆ»ģŗĻĀ±»ÆĮ×µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ ”£
4£©Į×ėę»ÆŗĻĪļŗ¬ÓŠ3ÖÖŌŖĖŲ£¬ĒŅ·Ö×ÓÖŠŌ×Ó×ÜŹżŠ”ÓŚ20”£0.10mol PCl5ŗĶ0.10mol NH4ClĒ”ŗĆĶźČ«·“Ó¦£¬Éś³ÉĀČ»ÆĒāŗĶ0.030molĮ×ėę»ÆŗĻĪļ”£ĶĘĖćĮ×ėę»ÆŗĻĪļµÄĻą¶Ō·Ö×ÓÖŹĮæ£ØĢįŹ¾£ŗM>300£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ( )
A.Ļņŗ¬ÓŠCaCO3³ĮµķµÄĖ®ÖŠĶØČėCO2 ÖĮ³ĮµķĒ”ŗĆČܽā£¬ŌŁĻņČÜŅŗÖŠ¼ÓČėNaHCO3±„ŗĶČÜŅŗ£¬ÓÖÓŠCaCO3³ĮµķÉś³É
B.ĻņNa2CO3ČÜŅŗÖŠÖšµĪ¼ÓČėµČĪļÖŹµÄĮæµÄĻ”ŃĪĖį£¬Éś³ÉµÄCO2ÓėŌNa2CO3µÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć2
C.µČÖŹĮæµÄNaHCO3ŗĶNa2CO3·Ö±šÓė×ćĮæŃĪĖį·“Ó¦£¬ŌŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬Éś³ÉµÄCO2Ģå»żĻąĶ¬
D.ĻņNa2CO3±„ŗĶČÜŅŗÖŠĶØČėCO2£¬ÓŠNaHCO3½į¾§Īö³ö
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃĢĘųÖŠNOxŹĒNOŗĶNO2µÄ»ģŗĻĪļ(²»ŗ¬N2O4)”£
(1)øł¾Ż·ĻĘųÅŷűź×¼£¬1 m3ŃĢĘų×īøßŌŹŠķŗ¬400 mg NOx”£ČōNOxÖŠNOÖŹĮæ·ÖŹżĪŖ0.85£¬Ōņ1 m3ŃĢĘųÖŠ×īøßŌŹŠķŗ¬NO___________________L(±ź×¼×“æö£¬±£Įō2Ī»Š”Źż)”£
(2)¹¤ŅµÉĻĶس£ÓĆČÜÖŹÖŹĮæ·ÖŹżĪŖ0.150µÄNa2CO3Ė®ČÜŅŗ(ĆܶČ1.16 g”¤mL-1)×÷ĪŖNOxĪüŹÕ¼Į£¬ŌņĢ¼ĖįÄĘČÜŅŗĪļÖŹµÄĮæÅضČĪŖ__________________________mol”¤L-1(±£Įō2Ī»Š”Źż)”£
(3)ŅŃÖŖ£ŗNO+NO2+Na2CO3 2NaNO2+CO2¢Ł
2NaNO2+CO2¢Ł
2NO2+Na2CO3 NaNO2+NaNO3+CO2¢Ś
NaNO2+NaNO3+CO2¢Ś
1 m3ŗ¬2 000 mg NOxµÄŃĢĘųÓĆÖŹĮæ·ÖŹżĪŖ0.150µÄĢ¼ĖįÄĘČÜŅŗĪüŹÕ”£ČōĪüŹÕĀŹĪŖ80%£¬ĪüŹÕŗóµÄŃĢĘų_______________Åŷűź×¼(Ģī”°·ūŗĻ”±»ņ”°²»·ūŗĻ”±)£¬ĄķÓÉ£ŗ_________________”£
(4)¼ÓČėĻõĖįæÉøıäŃĢĘųÖŠNOŗĶNO2µÄ±Č£¬·“Ó¦ĪŖ£ŗNO+2HNO3 3NO2+H2O
3NO2+H2O
µ±ŃĢĘųÖŠn(NO)”Ćn(NO2)=2”Ć3Ź±£¬ĪüŹÕĀŹ×īøß”£
1 m3ŃĢĘųŗ¬2 000 mg NOx£¬ĘäÖŠn(NO)”Ćn(NO2)=9”Ć1”£
¼ĘĖć£ŗ(¢”)ĪŖĮĖ“ļµ½×īøßĪüŹÕĀŹ£¬1 m3ŃĢĘųŠčÓĆĻõĖįµÄĪļÖŹµÄĮæ(±£Įō3Ī»Š”Źż)”£
(¢¢)1 m3ŃĢĘų“ļµ½×īøßĪüŹÕĀŹ90%Ź±£¬ĪüŹÕŗóÉś³ÉNaNO2µÄÖŹĮæ(¼ŁÉčÉĻŹöĪüŹÕ·“Ó¦ÖŠ£¬·“Ó¦¢Ł±Č·“Ó¦¢ŚŃøĖŁ”£¼ĘĖć½į¹ū±£Įō1Ī»Š”Źż)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³Ń§Ļ°Š”×éĢ½¾æÅØ”¢Ļ”ĻõĖįŃõ»ÆŠŌµÄĻą¶ŌĒæČõ£¬°“ĻĀĶ¼×°ÖĆ½ųŠŠŹµŃé£Ø¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©”£ŹµŃé±ķĆ÷ÅØĻõĖįÄܽ«NOŃõ»Æ³ÉNO2£¬¶ųĻ”ĻõĖį²»ÄÜŃõ»ÆNO”£ÓÉ“ĖµĆ³öµÄ½įĀŪŹĒÅØĻõĖįµÄŃõ»ÆŠŌĒæÓŚĻ”ĻõĖį”£
æÉŃ”Ņ©Ę·£ŗÅØĻõĖį”¢3 mol/LĻ”ĻõĖį”¢ÕōĮóĖ®”¢ÅØĮņĖį”¢ĒāŃõ»ÆÄĘČÜŅŗ¼°¶žŃõ»ÆĢ¼”£ŅŃÖŖ£ŗĒāŃõ»ÆÄĘČÜŅŗ²»ÓėNO·“Ó¦£¬ÄÜÓėNO2·“Ó¦”£
2NO2+2NaOH====NaNO3+NaNO2+H2O
£Ø1£©ŹµŃéÓ¦±ÜĆāÓŠŗ¦ĘųĢåÅŷŵ½æÕĘųÖŠ”£×°ÖĆ¢Ū¢Ü¢ŽÖŠŹ¢·ÅµÄŅ©Ę·ŅĄ“ĪŹĒ___________”£
£Ø2£©µĪ¼ÓÅØĻõĖįÖ®Ē°µÄ²Ł×÷ŹĒ¼ģŃé×°ÖƵÄĘųĆÜŠŌ£¬¼ÓČėŅ©Ę·£¬“ņæŖµÆ»É¼Šŗó___________”£
£Ø3£©×°ÖĆ¢ŁÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________”£
£Ø4£©×°ÖĆ¢ŚµÄ×÷ÓĆŹĒ___________£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________”£
£Ø5£©øĆŠ”×éµĆ³öµÄ½įĀŪĖłŅĄ¾ŻµÄŹµŃéĻÖĻóŹĒ___________”£
£Ø6£©ŹµŃé½įŹųŗó£¬Ķ¬Ń§ĆĒ·¢ĻÖ×°ÖĆ¢ŁÖŠČÜŅŗ³ŹĀĢÉ«£¬¶ų²»ĻŌĄ¶É«”£¼×Ķ¬Ń§ČĻĪŖŹĒøĆČÜŅŗÖŠĻõĖįĶµÄÖŹĮæ·ÖŹż½ĻøßĖłÖĀ£¬¶ųŅŅĶ¬Ń§ČĻĪŖŹĒøĆČÜŅŗÖŠČܽāĮĖÉś³ÉµÄĘųĢ唣Ķ¬Ń§ĆĒ·Ö±šÉč¼ĘĮĖŅŌĻĀ4øöŹµŃéĄ“ÅŠ¶ĻĮ½ÖÖæ“·ØŹĒ·ńÕżČ·”£ÕāŠ©·½°øÖŠæÉŠŠµÄŹĒ£ØŃ”ĢīŠņŗÅ×ÖÄø£©___________”£
a.¼ÓČČøĆĀĢÉ«ČÜŅŗ£¬¹Ū²ģŃÕÉ«±ä»Æ
b.¼ÓĖ®Ļ”ŹĶøĆĀĢÉ«ČÜŅŗ£¬¹Ū²ģĀĢÉ«±ä»Æ
c.ĻņøĆĀĢÉ«ČÜŅŗÖŠĶØČėµŖĘų£¬¹Ū²ģŃÕÉ«±ä»Æ
d.Ļņ±„ŗĶĻõĖįĶČÜŅŗÖŠĶØČėÅØĻõĖįÓėĶ·“Ó¦²śÉśµÄĘųĢ壬¹Ū²ģŃÕÉ«±ä»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼±ķŹ¾Ä³¹ĢĢ¬µ„ÖŹA¼°Ęä»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£ØijŠ©²śĪļŗĶ·“Ó¦Ģõ¼žŅŃĀŌČ„£©”£»ÆŗĻĪļBŌŚ³£ĪĀ³£Ń¹ĻĀĪŖĘųĢ壬BŗĶCµÄĻą¶Ō·Ö×ÓÖŹĮæÖ®±ČĪŖ4£ŗ5£¬»ÆŗĻĪļDŹĒÖŲŅŖµÄ¹¤ŅµŌĮĻ”£
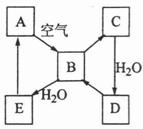
£Ø1£©Š“³öAŌŚ¼ÓČČĢõ¼žĻĀÓėH2·“Ó¦µÄ»Æѧ·½³ĢŹ½
£Ø2£©Š“³öEÓėAµÄĒā»ÆĪļ·“Ӧɜ³ÉAµÄ»Æѧ·½³ĢŹ½
£Ø3£©Š“³öŅ»øöÓÉDÉś³ÉBµÄ»Æѧ·½³ĢŹ½ ;
£Ø4£©½«5mL0.10mol”¤L-1µÄEČÜŅŗÓė10mL0.10mol”¤L-1µÄNaOHČÜŅŗ»ģŗĻ”£
¢ŁŠ“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½ ;
¢Ś·“Ó¦ŗóČÜŅŗµÄpH 7£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©£¬ĄķÓÉŹĒ ;
¢Ū¼ÓČČ·“Ó¦ŗóµÄČÜŅŗ£¬ĘäpH £ØĢī ”°Ōö“ó”±”¢”°²»±ä”±»ņ”°¼õŠ””±£©£¬ĄķÓÉŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
U”¢V”¢W”¢X”¢Y”¢ZŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĮłÖÖ³£¼ūŌŖĖŲ”£YµÄµ„ÖŹŌŚW2ÖŠČ¼ÉյIJśĪļæÉŹ¹Ę·ŗģČÜŅŗĶŹÉ«”£ZŗĶWŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļZ3W4¾ßÓŠ“ÅŠŌ”£UµÄµ„ÖŹŌŚW2ÖŠČ¼ÉÕæÉÉś³ÉUWŗĶUW2Į½ÖÖĘųĢ唣X µÄµ„ÖŹŹĒŅ»ÖÖ½šŹō£¬øĆ½šŹōŌŚUW2ÖŠ¾ēĮŅČ¼ÉÕÉś³ÉŗŚ”¢°×Į½ÖÖ¹ĢĢ唣
µÄµ„ÖŹŹĒŅ»ÖÖ½šŹō£¬øĆ½šŹōŌŚUW2ÖŠ¾ēĮŅČ¼ÉÕÉś³ÉŗŚ”¢°×Į½ÖÖ¹ĢĢ唣
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©VµÄµ„ÖŹ·Ö×ӵĽį¹¹Ź½ĪŖ £»XWµÄµē×ÓŹ½ĪŖ £»ZŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ ”£
£Ø2£©UŌŖĖŲŠĪ³ÉµÄĶ¬ĖŲŅģŠĪĢåµÄ¾§ĢåĄąŠĶæÉÄÜŹĒ£ØĢīŠņŗÅ£© ”£
¢ŁŌ×Ó¾§Ģå ¢ŚĄė×Ó¾§Ģå ¢Ū·Ö×Ó¾§Ģå ¢Ü½šŹō¾§Ģå
£Ø3£©U”¢V”¢WŠĪ³ÉµÄ10µē×ÓĒā»ÆĪļÖŠ£¬U”¢VµÄĒā»ÆĪļ·Šµć½ĻµĶµÄŹĒ£ØŠ“»ÆѧŹ½£©
£»V”¢WµÄĒā»ÆĪļ·Ö×Ó½įŗĻH+ÄÜĮ¦½ĻĒæµÄŹĒ£ØŠ“»ÆѧŹ½£© £»ÓĆŅ»øöĄė×Ó·½³ĢŹ½¼ÓŅŌÖ¤Ć÷ ”£
£Ø4£©YW2ĘųĢåĶØČėBaCl2ŗĶHNO3µÄ»ģŗĻČÜŅŗ£¬Éś³É°×É«³ĮµķŗĶĪŽÉ«ĘųĢåVW£¬ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬ÓÉ“ĖæÉÖŖVWŗĶYW2»¹ŌŠŌ½ĻĒæµÄŹĒ£ØŠ“»ÆѧŹ½£© ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com