
��ҵ�ϰ����Ĵ���������ȡ����Ļ���.��һ���¶��½�4mol NH3��4mol O2������������2L�ܱ�������,�������·�Ӧ:4NH3(g)+5O2(g)  4NO(g)+6H2O(g)��
4NO(g)+6H2O(g)��
��H<0,2����ĩ������1.2molH2O,��:
(1)��H2O��ʾ�ķ�Ӧ����Ϊ________mol/(L?min).
(2)O2��2����ĩ��Ũ��Ϊ_______mol /L.
(3)�жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��______(����ĸ).
a.NH3��NOŨ����� b.NO�ٷֺ������ֲ���
c.�����������ѹǿ���� d.NO������������NH3�������������
e.�����л��������ܶȱ��ֲ��� f.O2���������ٸı�
(4)�����NH3��ת����,���д�ʩ���е��� (����ĸ).
a.��װ�����ٳ���O2 b.�ı����
c.����ѹǿ d.����¶�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
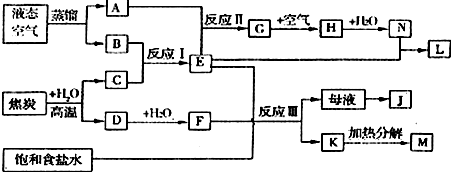
 ��ҵ�����ð�����������һϵ�з�Ӧ�����Ʊ����ᣮ
��ҵ�����ð�����������һϵ�з�Ӧ�����Ʊ����ᣮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϰ����Ĵ���������ȡ����Ļ���.��һ���¶��½�4mol NH3��4mol O2������������2L�ܱ�������,�������·�Ӧ:4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)��
4NO(g)+6H2O(g)��
��H<0,2����ĩ������1.2molH2O,��:
(1)��H2O��ʾ�ķ�Ӧ����Ϊ________mol/(L•min).
(2)O2��2����ĩ��Ũ��Ϊ_______mol /L.
(3)�жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��______(����ĸ).
a.NH3��NOŨ����� b.NO�ٷֺ������ֲ���
c.�����������ѹǿ���� d.NO������������NH3�������������
e.�����л��������ܶȱ��ֲ��� f.O2���������ٸı�
(4)�����NH3��ת����,���д�ʩ���е��� (����ĸ).
a.��װ�����ٳ���O2 b.�ı����
c.����ѹǿ d.����¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���и߶�����3�·��¿���ѧ�Ծ� ���ͣ������
��ҵ�ϰ����Ĵ���������ȡ����Ļ���.��һ���¶��½�4mol NH3��4mol O2������������2L�ܱ�������,�������·�Ӧ:4NH3(g)+5O2(g)
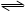 4NO(g)+6H2O(g)��
4NO(g)+6H2O(g)��
��H<0,2����ĩ������1.2molH2O,��:
(1)��H2O��ʾ�ķ�Ӧ����Ϊ________mol/(L•min).
(2)O2��2����ĩ��Ũ��Ϊ_______mol /L.
(3)�жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��______(����ĸ).
a.NH3��NOŨ����� b.NO�ٷֺ������ֲ���
c.�����������ѹǿ���� d.NO������������NH3�������������
e.�����л��������ܶȱ��ֲ��� f.O2���������ٸı�
(4)�����NH3��ת����,���д�ʩ���е��� (����ĸ).
a.��װ�����ٳ���O2 b.�ı����
c.����ѹǿ d.����¶�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com