
ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ
2H2O(l)===2H2(g)£«O2(g) ¦¤H£½571.6 kJ·mol£1
2H2(g)£«O2(g)===2H2O(g) ¦¤H£½£483.6 kJ·mol£1
µ±1 gŅŗĢ¬Ė®±äĪŖĘųĢ¬Ė®Ź±£¬¶ŌĘäČČĮæ±ä»ÆµÄĻĀĮŠĆčŹö£ŗ¢Ł·ÅČČ£»¢ŚĪüČČ£»¢Ū2.44 kJ£»
¢Ü4.88 kJ£»¢Ż88 kJ”£ĘäÖŠÕżČ·µÄŹĒ(””””)
A£®¢ŚŗĶ¢Ż B£®¢ŁŗĶ¢Ū C£®¢ŚŗĶ¢Ü D£®¢ŚŗĶ¢Ū

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²»½ö»Æ¹¤Éś²śÓ¦×ńŃĀĢÉ«»ÆѧµÄŌŌņ,»ÆѧŹµŃéŅ²Ó¦×ńŃĀĢÉ«»ÆѧµÄŌŌņ,ŹµĻÖŌĮĻŗĶ¹ż³ĢµÄĀĢÉ«»Æ”£ĻĀĮŠŹµŃé»ņŹµŃé¹ż³Ģ×ńŃĀĢÉ«»ÆѧŌŌņµÄŹĒ(””””)
¢ŁŌŚŻĶČ”²Ł×÷µÄŃŻŹ¾ŹµŃéÖŠ,½«CCl4ŻĶČ”äåĖ®øÄĪŖCCl4ŻĶČ”µāĖ®
¢ŚŌŚĶÓėÅØĻõĖį·“Ó¦µÄŹµŃéÖŠ,½«ĶʬøÄĪŖæɵ÷½Śø߶ȵÄĶĖæ
¢Ū½«ŹµŃéŹŅµÄ·ĻĖįŅŗŗĶ·Ļ¼īŅŗÖŠŗĶŗóŌŁÅÅ·Å
¢ÜÓĆĖ«ŃõĖ®“śĢęøßĆĢĖį¼ŲÖĘŃõĘų
¢Ż½«ÓĆĶ·ŪÓėÅØĮņĖį·“Ó¦ÖĘČ”ĮņĖįĶµÄŹµŃé·½°øøÄĪŖĻČ½«Ķ·ŪŌŚæÕĘųÖŠ³ä·Ö¼ÓČČÖʵĆŃõ»ÆĶ,ŌŁ½«Ńõ»ÆĶČܽāŌŚĻ”ĮņĖįÖŠ
A.¢Ł¢Ś B.¢Ś¢Ū C.¢Ū¢Ü D.¢Ł¢Ś¢Ū¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃĒĀČĖįÄĘ(NaClO2)ŹĒŅ»ÖÖÖŲŅŖµÄĻū¶¾¼Į”£ŅŃÖŖ£ŗ¢ŁNaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ŹŹµ±Ģõ¼žĻĀæɽį¾§Īö³öNaClO2”¤3H2O£¬¢ŚClO2µÄ·ŠµćĪŖ283K£¬“æClO2Ņ×·Ö½ā±¬ÕØ£¬¢ŪHClO2ŌŚ25”ꏱµÄµēĄė³Ģ¶ČÓėĮņĖįµÄµŚ¶ž²½µēĄė³Ģ¶ČĻąµ±£¬æÉŹÓĪŖĒæĖį”£ČēĶ¼ŹĒ¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄĘµÄ¹¤ŅÕĮ÷³ĢĶ¼£ŗ
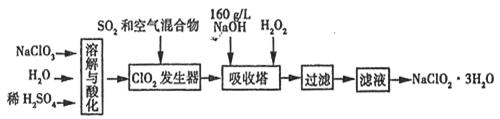
(1)C1O2·¢ÉśĘ÷ÖŠĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬·¢ÉśĘ÷ÖŠ¹ÄČėæÕĘųµÄ×÷ÓĆæÉÄÜŹĒ (Ń”ĢīŠņŗÅ)”£
A£®½«SO2Ńõ»Æ³ÉSO3ŌöĒæĖįŠŌ B£®Ļ”ŹĶC1O2ŅŌ·ĄÖ¹±¬ÕØ
C£®½«NaClO3Ńõ»Æ³ÉC1O2
(2)ŌŚøĆŹµŃéÖŠÓĆÖŹĮæÅØ¶ČĄ“±ķŹ¾NaOHČÜŅŗµÄ×é³É£¬ČōŹµŃ鏱ŠčŅŖ450ml
l60g£ÆLµÄNaOHČÜŅŗ£¬ŌņŌŚ¾«Č·ÅäÖĘŹ±£¬ŠčŅŖ³ĘČ”NaOHµÄÖŹĮæŹĒ g£¬
ĖłŹ¹ÓƵÄŅĒĘ÷³żĶŠÅĢĢģĘ½”¢ĮæĶ²”¢ÉÕ±”¢²£Į§°ōĶā£¬»¹±ŲŠėÓŠ
(3) ĪüŹÕĖžÄŚµÄĪĀ¶Č²»Äܳ¬¹ż20”ę£¬ĘäÖ÷ŅŖÄæµÄŹĒ _£¬ĪüŹÕĖžÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
(4)ŌŚĪüŹÕĖžÖŠ£¬æÉ“śĢęH2O2µÄŹŌ¼ĮŹĒ (ĢīŠņŗÅ)”£
A£®Na2O2 B£®Na2S C£®FeCl2 D£®KMnO4
(5)“ÓĀĖŅŗÖŠµĆµ½NaClO2”¤3H2O¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“ĪŹĒ £ØĢī²Ł×÷Ćū³Ę£©
A£®ÕōĮó B£®Õō·¢ÅØĖõ C£®×ĘÉÕ D£®¹żĀĖ E”¢ĄäČ“½į¾§
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄÜŌ“ĪŹĢāŹĒČĖĄąÉē»įĆęĮŁµÄÖŲ“óæĪĢā£¬H2”¢CO”¢CH3OH¶¼ŹĒÖŲŅŖµÄÄÜŌ“ĪļÖŹ£¬ĖüĆĒµÄČ¼ÉÕČČŅĄ“ĪĪŖ£285.8 kJ·mol£1”¢£282.5 kJ·mol£1”¢£726.7 kJ·mol£1”£ŅŃÖŖCOŗĶH2ŌŚŅ»¶ØĢõ¼žĻĀæÉŅŌŗĻ³É¼×“¼CO(g)£«2H2(g)===CH3OH(l)”£ŌņCOÓėH2·“Ó¦ŗĻ³É¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ(””””)
A£®CO(g)£«2H2(g)===CH3OH(l) ¦¤H£½£127.4 kJ·mol£1
B£®CO(g)£«2H2(g)===CH3OH(l) ¦¤H£½127.4 kJ·mol£1
C£®CO(g)£«2H2(g)===CH3OH(g) ¦¤H£½£127.4 kJ·mol£1
D£®CO(g)£«2H2(g)===CH3OH(g) ¦¤H£½127.4 kJ·mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ±ä»ÆÖŠ£¬ŹōÓŚĪüČČ¹ż³ĢµÄŹĒ(””””)
¢ŁŅŗĢ¬Ė®Ęū»Æ””¢Ś½«µØ·Æ¼ÓČȱäĪŖ°×É«·ŪÄ©””¢ŪÅØĮņĖįĻ”ŹĶ””¢ÜĀČĖį¼Ų·Ö½āÖĘŃõĘų””
¢ŻÉśŹÆ»ŅøśĖ®·“Ӧɜ³ÉŹģŹÆ»Ņ
””””””””””””””””””””””””””””””””””””””
A£®¢Ł¢Ü B£®¢Ś¢Ū C£®¢Ł¢Ü¢Ż D£®¢Ł¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅĄ¾ŻŠšŹö£¬Š“³öĻĀĮŠ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½”£
(1)ČōŹŹĮæµÄN2ŗĶO2ĶźČ«·“Ó¦£¬ĆæÉś³É23 g NO2ŠčŅŖĪüŹÕ16.95 kJČČĮ攣ĘäČČ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________
________________________________________________________________________ӣ
(2)ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌŚC2H2(ĘųĢ¬)ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®µÄ·“Ó¦ÖŠ£¬ĆæÓŠ5NAøöµē×Ó×ŖŅĘŹ±£¬·Å³ö650 kJµÄČČĮ攣ĘäČČ»Æѧ·½³ĢŹ½ĪŖ
________________________________________________________________________
________________________________________________________________________ӣ
(3)ŅŃÖŖ²šæŖ1 mol H—H¼ü”¢1 mol N—H¼ü”¢1 mol N”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436 kJ”¢
391 kJ”¢946 kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ
________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æŲÖĘŹŹŗĻµÄĢõ¼ž£¬½«·“Ó¦2Fe3£«£«2I£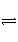
 2Fe2£«£«I2Éč¼Ę³ÉČēĻĀĶ¼ĖłŹ¾µÄŌµē³Ų”£ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ(””””)
2Fe2£«£«I2Éč¼Ę³ÉČēĻĀĶ¼ĖłŹ¾µÄŌµē³Ų”£ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ(””””)

A£®·“Ó¦æŖŹ¼Ź±£¬ŅŅÖŠŹÆÄ«µē¼«ÉĻ·¢ÉśŃõ»Æ·“Ó¦
B£®·“Ó¦æŖŹ¼Ź±£¬¼×ÖŠŹÆÄ«µē¼«ÉĻµÄFe3£«±»»¹Ō
C£®µēĮ÷¼Ę¶ĮŹżĪŖĮ揱£¬·“Ó¦“ļµ½»ÆŃ§Ę½ŗāדĢ¬
D£®µēĮ÷¼Ę¶ĮŹżĪŖĮćŗó£¬ŌŚ¼×ÖŠČÜČėFeCl2¹ĢĢ壬ŅŅÖŠµÄŹÆÄ«µē¼«ĪŖøŗ¼«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ1 gĒāĘųĶźČ«Č¼ÉÕÉś³ÉĖ®ÕōĘųŹ±·Å³öČČĮæ121 kJ£¬ĒŅŃõĘųÖŠ1 mol O£½O¼üĶźČ«¶ĻĮŃŹ±ĪüŹÕČČĮæ496 kJ£¬Ė®ÕōĘųÖŠ1 mol H”ŖO¼üŠĪ³ÉŹ±·Å³öČČĮæ463 kJ£¬ŌņĒāĘųÖŠ1 mol H”ŖH¼ü¶ĻĮŃŹ±ĪüŹÕČČĮæĪŖ( )
A£®920 kJ B£®557 kJ C£®436 kJ D£®188 kJ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻņAgCl±„ŗĶČÜŅŗÖŠµĪ¼Ó×ćĮæNaBrČÜŅŗ£¬²śÉśµ»ĘÉ«³Įµķ£¬ŌŁĻņČÜŅŗÖŠµĪ¼ÓKIČÜŅŗ£¬ÓÖ²śÉś»ĘÉ«³Įµķ”£ÓŠ¹ŲĻĀĮŠĖµ·ØÕżČ·µÄŹĒ( )
A£®²śÉśµÄµ»ĘÉ«³ĮµķĪŖAgI
B£®²śÉśµÄ»ĘÉ«³ĮµķĪŖAgBr
C£®ÓÉÉĻŹöŹµŃéĻÖĻóæÉĖµĆ÷Čܽā¶ČS(AgBr)>S(AgI)>S(AgCl)
D£®ÉĻŹöŹµŃéĻÖĻóĖµĆ÷³ĮµķÖ®¼äæÉĻą»„×Ŗ»Æ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com