
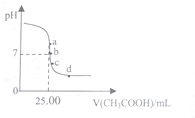
 �����£���25.00mL0.1000mol•L-1��NaOH��Һ����ε���0.1000mol•L-1CH3COOH��Һ��PH���߱仯��ͼ��ʾ��������˵��һ����ȷ���ǣ�������
�����£���25.00mL0.1000mol•L-1��NaOH��Һ����ε���0.1000mol•L-1CH3COOH��Һ��PH���߱仯��ͼ��ʾ��������˵��һ����ȷ���ǣ�������| A�� | a�㣺c��CH3COONa��=0.1000 mol•L-1 | B�� | b�㣺c��CH3COONa��=c��CH3COOH�� | ||
| C�� | c�㣺c��H+��=c��CH3COO-��+c��CH3COOH�� | D�� | d�㣺c��Na+����c��CH3COO-��+c��CH3COOH�� |
���� ��25mL 0.1mol��L-1NaOH��Һ����μ���0.1mol•L-1 CH3COOH ��Һ������֮�����Ӧ����ǡ����ȫ��Ӧʱ�������������Ϊ25mL������Ӧ����Һ������ʱ������Ӧ�Թ�������c��OH-��=c��H+����ע����ݵ���غ�˼�����Ƚ�����Ũ�ȴ�С��
A����a�㣬��25mL 0.1mol��L-1NaOH��Һ����μ���0.1mol•L-1 CH3COOH ��Һ25ml��ǡ�÷�Ӧ���ɴ�������Һ�ʼ��ԣ�
B����b����Һ�����ԣ�������c��OH-��=c��H+�������ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-������һ����c��Na+��=c��CH3COO-������Һ�ijɷ�Ϊ����Ӧ���ɵĴ����ƺ�ʣ��Ĵ��ᣬ�����Ƶ�ˮ��̶Ⱥʹ���ĵ���̶���ȣ�
C����c�㣬��Һ�����ԣ�����c��OH-����c��H+�������ݵ���غ㣺c��Na+��+c��H+��=c��CH3COO-��+c��OH-���������غ�c��CH3COO-��+c��CH3COOH��=0.1mol•L-1=2c��Na+�������������غ�͵���غ���������
D����d��ʱ������ʣ�࣬��Һ�ijɷ�Ϊ����Ӧ���ɵĴ����ƺ�ʣ��Ĵ��ᣮ
��� �⣺A����a�㣬��25mL 0.1mol��L-1NaOH��Һ����μ���0.1mol•L-1 CH3COOH ��Һ25ml��ǡ�÷�Ӧ���ɴ�������Һ�ʼ��ԣ�c��CH3COONa��=0.05000 mol•L-1��
��A����
B����b����Һ�����ԣ�������c��OH-��=c��H+�������ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-������һ����c��Na+��=c��CH3COO-������Һ�ijɷ�Ϊ����Ӧ���ɵĴ����ƺ�ʣ��Ĵ��ᣬ�����Ƶ�ˮ��̶Ⱥʹ���ĵ���̶���ȣ����У�c��CH3COONa����c��CH3COOH������B����
C����c�㣬��Һ�����ԣ�����c��OH-����c��H+�������ݵ���غ㣺c��Na+��+c��H+��=c��CH3COO-��+c��OH-���������غ�c��CH3COO-��+c��CH3COOH��=0.1mol•L-1=2c��Na+����c��CH3COOH��+2c��H+��=c��CH3COO-��+2c��OH-������C����
D����d��ʱ������ʣ�࣬��Һ�ijɷ�Ϊ����Ӧ���ɵĴ����ƺ�ʣ��Ĵ��ᣬ��Һ��ֻ��������������Na+��H+��CH3COO-��OH-���õ�c��Na+����c��CH3COO-��+c��CH3COOH������D��ȷ��
��ѡD��
���� ������һ������Ϻ�����Ũ�ȴ�С�Ƚϵ���Ŀ��Ҫ�����ÿ��״̬ʱ��Һ������������ע������غ�˼���������ã���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֪A-+H2B��������=HA+HB-������H+��������HB-��A-��B2- | |
| B�� | ��25��ʱ����cmol/L�Ĵ�����Һ��0.02mol/LNaOH��Һ�������Ϻ���Һǡ�ó����ԣ��ú�c�Ĵ���ʽ��ʾCH3COOH�ĵ��볣��Ka=2��10-9/��c-0.02�� | |
| C�� | ����CO2 ͨ��0.1 mol/LNaOH��Һ������Һ���ԣ�����Һ��2 c��CO32����+c��HCO3����=0.1 mol/L | |
| D�� | �����£�����ͬ�����pH=3�����pH=11һԪ��BOH��Һ��ϣ�������Һ����Ϊ����Ҳ����Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SnH4��GeH4��SiH4��CH4 | B�� | SbH3��AsH3��PH3��NH3 | ||
| C�� | HI��HBr��HCl��HF | D�� | H2Te��H2Se��H2S��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ɫ�Լ�ƿʢ��Ũ���� | B�� | ����������ʢ��Ũ���� | ||
| C�� | �ò����Լ�ƿʢ������� | D�� | �ô��������Լ�ƿʢ�ű� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ʵ�����в����������ȡ������ʵ��װ����ͼ��ʾ��ʡ�Լӳֺ;���װ�ã���c�������ж���ȷ���ǣ�������
ʵ�����в����������ȡ������ʵ��װ����ͼ��ʾ��ʡ�Լӳֺ;���װ�ã���c�������ж���ȷ���ǣ�������| ѡ�� | �Լ�a | �Լ�b | �Լ�c | c�е����� |
| A | Ũ��ˮ | ��ʯ�� | ��������Һ | �ȳ�������ʧ |
| B | ϡ���� | �� | ˮ | �Թܿ����������� |
| C | Ũ���� | ������� | ʯ����Һ | ��Һ��ɫû�б仯 |
| D | ϡ���� | ���� | ��������Һ | �ȳ�������ʧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
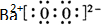 ��д����ʽ����
��д����ʽ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ��ɳ�и�����ѧ�ڵ�13���ܲ������ۺϻ�ѧ�Ծ� ���ͣ�ʵ����
��ʽ̼���ܣ�Co4��OH��,��CO3��4�ݳ��������Ӳ��ϡ����Բ��ϵ����Ӽ���������ˮ������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ��ʾ��װ�ã����������������顣
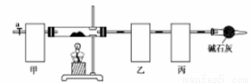
ʵ�鲽�����£�
�ٳ�ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ������ҡ���װ�õ�������
�ڰ���ͼ��ʾװ����װ������������ ��
�ۼ���Ӳ�ʲ����ܣ�����װ���� ������ֹͣ���ȣ�
�ܴ���a������ͨ����������Ӻ����ҡ���װ�õ�������
�ݼ��㡣
��1��������ͼʾѡ��������װ�����ڷ����У�ʹ����ʵ��װ��������ѡ����ĸ��ţ����ظ�ѡ��
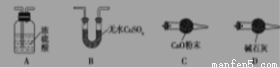
�ף� �ң� ����
��װ�õ������� ��
��2���������ʡ�Ե�ʵ�����Ϊ ��
���������װ�õ�����Ϊ ��
������л���ͨ����������ӵ�Ŀ���� ��
��3��������ȷװ�ý���ʵ�飬����������ݡ�
��װ�õ�����/g | ��װ�õ�����/g | |
����ǰ | 80.00 | 62.00 |
���Ⱥ� | 80.36 | 62.88 |
��ü�ʽ̼���ܵĻ�ѧʽΪ_____________��
��4��CO2��SO2��Ϊ�������壬�������ơ�Ϊ�˱Ƚ��������̼�������ǿ����ijͬѧ������װ�ý���ʵ�顣
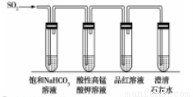
��д����ʵ���ܴﵽʵ��Ŀ�ĵ�ʵ������____________��
������SO2ͨ��ˮ�������ͣ������ʵ��֤�������������ᣬʵ�鷽��Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������ʡ�������и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ڳ�����pH��2�Ĵ��ᣬ����������ȷ����
A��c��CH3COOH����0��01mol��L��1
B��c��H������c��CH3COO����
C����ˮϡ��100������ ҺpH��4
ҺpH��4
D������CH3COONa���壬������CH3COOH�ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com