
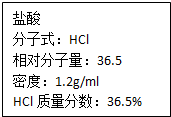
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺| 1000�� |
| M |
| n |
| V |
| 1000�� |
| M |
| 1000��1.2g/ml��36.5% |
| 36.5g/mol |
| n |
| V |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ҺA |
| ��ַ�Ӧ |
| ������ҺB |
| ��ַ�Ӧ |
| A����ҺA��B�������������NaOH��Һ |
| B����ҺA��B�����Ծ�ѡ��ϡ���� |
| C������ҺBѡ��Ũ���ᣬ����ͭ����������ƫ�� |
| D��ʵ���ҷ����������ʵʩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���ڢ� | C���٢� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���÷�Һ������ˮ�;ƾ� |
| B���������뱽���屽 |
| C���ù��˷����ᴿ������ |
| D���ñ���̼��������Һ��ȥ������̼�л��е������Ȼ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ʵ�鷽�� | pH��ʵ������ |
| ���� | �����£�ȡ����������ʵ���Ũ�Ⱦ�Ϊ0.1mol��L-1������������ᣬ�ֱ��������������ͬ����ɰֽ��ĥ����þ�����ռ����������� | |
| ���� | ��SO3��g����SO2��g���ֱ�ͨ��ˮ�У�����������������Һ��pH | ���������pHС�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com