
������ϩ��ij���Ļ�����壬ijͬѧ��Ҫ�о������ķ���ʽ�����ڳ���ʱ��һ�������Ļ������ȼ�գ����ȼ�ղ���������������Ƿ�Ӧǰ�����������2.86������ͨ������ó��˸���̬���ķ���ʽ�����������ķ����������̣���������пհף�
(1)������������ƽ������ʽΪCxHy���������֪
 ��2.86����
��2.86����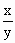 ��________��
��________��
(2)������ϩ���⡢̼ԭ�ӱ�Ϊ________���ɴ˿�֪��δ֪��ֻ��������________(���)��
(3)���δ֪���ķ���ʽΪ________���г�����ʽ________����ø���Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com