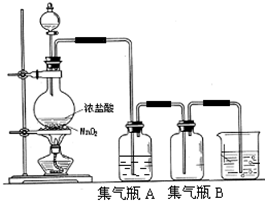
ʵ�������������������������з�����ȡ�ģ�
4HCl��Ũ��+MnO
2Cl
2��+MnCl
2+2H
2O
�Իش��������⣺
��1���÷�Ӧ��������ԭ��Ӧ��______�������������ԭ��Ӧ����ָ��______����������______�ǻ�ԭ����д���÷�Ӧ�����ӷ���ʽ______��
��2������ƿA��ʢװ���DZ���ʳ��ˮ��ע�������ڱ���ʳ��ˮ���ܽ�Ⱥ�С�����Ȼ����ڱ���ʳ��ˮ�е��ܽ����ܴ���������______��
��3����������ˮ�����ԣ��������ж������о��ҵĴ̼��ԣ�������������������ж���������������β��ֱ����������У�����Ⱦ������ʵ�����пɲ���______��Һ�������ж���������
��4��һ��ʵ���У���Ũ����80mL�����ܶ�Ϊ1.19g?cm-
3��HCl����������Ϊ36.5%����MnO
2ǡ����ȫ��Ӧ��������5.6L����״���£����������Լ���Ũ��������ʵ���Ũ�Ⱥͱ�������HCl�����ʵ�����



 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
 ʵ�������������������������з�����ȡ�ģ�
ʵ�������������������������з�����ȡ�ģ� ʵ�������������������������з�����ȡ�ģ�
ʵ�������������������������з�����ȡ�ģ�

 Cl2����MnCl2��2H2O��
Cl2����MnCl2��2H2O��