
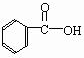
 ��
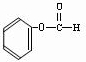
��
 ��
��
| 2.16g |
| 18g/mol |
| 12.32g |
| 44g/mol |
| 4.88g |
| 122g/mol |
| 0.28mol |
| 0.04mol |
| 0.24mol |
| 0.04mol |
| 122-12��7-6 |
| 16 |
 ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)ȼ�մ˻�����3.24 mg�������������ٿˣ�
(2)��˻�����ķ���ʽ��
(3)�û�����1��������1����������д�������ڷӺʹ���ͬ���칹��Ľṹ��ʽ�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������C��H��O���л���3.24gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ��CaCl2�ܣ�A���ͼ�ʯ�ң�B�������A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108������
��1��ȼ�մ��л���3.24g������O2��������
��2������л���ķ���ʽ��
��3�������л���1��������1����������д������ͬ����ͬ���칹��Ľṹ��ʽ���֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ�߶��ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ�������
������C��H��O���л���3.24gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ��CaCl2�ܣ�A���ͼ�ʯ�ң�B�������A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108������
��1��ȼ�մ��л���3.24g������O2��������
��2������л���ķ���ʽ��
��3�������л���1��������1����������д������ͬ����ͬ���칹��Ľṹ��ʽ���֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ�걣���и߶��꼶�ڶ�ѧ������������ѧ�Ծ� ���ͣ������
��9�֣�������C��H��O���л���4.08g��װ��Ԫ�ط���װ�ã�ͨ��������O2ʹ����ȫȼ�գ������ɵ���������ͨ��ʢ���Ȼ��Ƶĸ����A��ʢ�м�ʯ�ҵĸ����B�����A������������2.16g��B������������10.56g����֪���л������Է�������Ϊ136��
��1��ȼ�մ˻�����4.08g�������������������� g
��2����˻�����ķ���ʽ
��3�������л��������ȷ������ܵĽṹ��ʽ��
�������л���Ϊ�����廯����䱽����ֻ��һ��ȡ��������������������������Һ��Ӧ������ṹ��ʽ����Ϊ���м���д���֣���
�������л����ܷ���������Ӧ�����ڳ���������Ũ��ˮ����ȡ����Ӧ���䱽����������ȡ����������ṹ��ʽ����Ϊ��дһ�֣���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com