
��11�֣���ͼ����ĸ�����������ʾ�Ϊ��ѧ��ѧ�������ʡ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�������C��D��HΪ���嵥�ʡ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�ء�Y�Ǻ��ɫ��������Щ������һ�������´�������ת����ϵ��������Щ��Ӧ����������Ѿ���ȥ���Իش��������⣺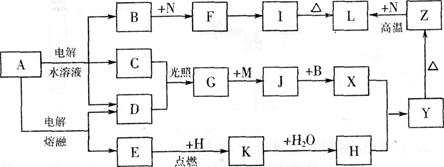
(1) ��ҵ�ϣ��ڵ��A��Һ���豸�н����������������� �����������豸���ƣ�
(2) д��A��ˮ��Һ�������ӷ���ʽ ��
(3) д��K��CO2��Ӧ�Ļ�ѧ����ʽ ��
(4) Y��NaClO��B�Ļ����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�����д���÷�Ӧ�����ӷ���ʽ ��
(5) һ������Z��N�Ļ�����Ϊ���ȷ֣�һ��ֱ������������������Һ��������Ϊamol����һ�ݸ����³�ַ�Ӧ�������ǹ����������ķ�Ӧ����Ĺ������������������������Ϊbmol������a:b=9:7����������Z��N�����ʵ���֮��Ϊ ��
 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| ����ø |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| C | D | G |
��ʼ���/mol | 6 | 4 | 0 |
ijʱ�����/mol | 3 | 3 | 2 |
(1)��X��M��N���ֶ�������ͬ����Ԫ����ɣ�Mԭ�ӵ����������������ڲ��������![]() ��NԪ��������������ĸ��۴�����Ϊ6����Ӧ�ܵ��������ϱ�����ʾ��
��NԪ��������������ĸ��۴�����Ϊ6����Ӧ�ܵ��������ϱ�����ʾ��
��д����Ӧ�ڵ����ӷ���ʽ________________________��
������G��ˮ��Һ������Ũ���ɴ�С��˳��Ϊ____________��
(2)��X��һ�ֺ������Σ���B��һ�ֹ���Ԫ�صĽ�������Ӧ������B��C�����ʵ���֮��Ϊ4��1����Ӧ�ڲ����ض��ķ�Ӧ�������ܽ��У���A��Ũ�Ȳ�ͬ������E��ͬ����X���ʵĻ�ѧʽ������_______________��
(3)��X��һ�ֺ������Σ�B�ǽ������ʣ�C��D�Ƿǽ������ʣ���Ӧ����ҪB��A��Ũ��Һ���Ȳ��ܽ��У������ɵ�E��G��ͬһ�����ʡ�
д����Ӧ�ٵĻ�ѧ����ʽ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����ڶ���������⻯ѧ�Ծ��������棩 ���ͣ������
��11�֣���ͼ����ĸ�����������ʾ�Ϊ��ѧ��ѧ�������ʡ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�������C��D��HΪ���嵥�ʡ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�ء�Y�Ǻ��ɫ��������Щ������һ�������´�������ת�� ��ϵ��������Щ��Ӧ����������Ѿ���ȥ���Իش��������⣺
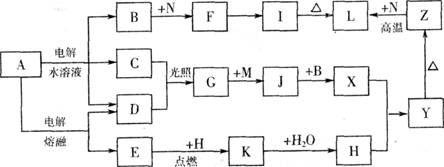
(1) ��ҵ�ϣ��ڵ��A��Һ���豸�н����������������� �����������豸���ƣ�
(2) д��A��ˮ��Һ�������ӷ���ʽ ��
(3) д��K��CO2��Ӧ�Ļ�ѧ����ʽ ��
(4) Y��NaClO��B�Ļ����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�����д���÷�Ӧ�����ӷ���ʽ ��
(5) һ������Z��N�Ļ�����Ϊ���ȷ֣�һ��ֱ������������������Һ��������Ϊamol����һ�ݸ����³�ַ�Ӧ�������ǹ����������ķ�Ӧ����Ĺ������������������������Ϊbmol������a:b=9:7����������Z��N�����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����߿�ģ�⣨�ģ����ۻ�ѧ�Ծ��������棩 ���ͣ������
(��15��)��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���塣����д���пհף�

��1����ͼ���������������ڷǵ���ʵ���������Ϊ ��
��2���õ���ʽ��ʾ��H���γɹ��� ��
��3����E��ˮ��Һ���ɲ����յõ��Ĺ������ʵĻ�ѧʽΪ ��
��4��F��ˮ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��5��F��ˮ��Һ�Լ��Ե�ԭ�������ӷ���ʽ��ʾ�� ��
��6��E��F��L�з�Ӧ�����ӷ���ʽΪ ��
��7��H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ .
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com