
|
ŅŃÖŖ£ŗH2(g)£«1/2O2(g)£½H2O(l)””¦¤H£½£285.83 kJ/mol CO(g)£«1/2O2(g)£½CO2(g)””¦¤H£½£282.9 kJ/mol ČōĒāĘųÓėŅ»Ńõ»ÆĢ¼µÄ»ģŗĻĘųĢåĶźČ«Č¼ÉÕæÉÉś³É5.4æĖĖ®(l)£¬²¢·Å³ö114.3 kJµÄČČĮ棬Ōņ»ģŗĻĘųÖŠŅ»Ńõ»ÆĢ¼µÄĪļÖŹµÄĮæĪŖ | |
| [””””] | |
A£® |
0.22 mol |
B£® |
0.15 mol |
C£® |
0.1 mol |
D£® |
0.05 mol |
 ѧ¶ųÓÅĻĪ½Ó½Ģ²ÄÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ѧ¶ųÓÅĻĪ½Ó½Ģ²ÄÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø
Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø ½š²©ŹæŅ»µćČ«ĶØĻµĮŠ“š°ø
½š²©ŹæŅ»µćČ«ĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 4 |
| 1 |
| 2 |
| 3 |
| 2 |
| 3 |
| 4 |
| 1 |
| 2 |
| 3 |
| 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| »Æѧ¼ü | ¼üÄÜ£ØkJ/mol£© | »Æѧ¼ü | ¼üÄÜ£ØkJ/mol£© |
| N”ŌN | 942 | H-O | 460 |
| N-H | 391 | O=O | 499 |
| H-H | 437 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌĢģ½ņ¾ķĄķ×Ū»Æѧ ĢāŠĶ£ŗ022
ijŹŠ¶Ō“óĘų½ųŠŠ¼ą²ā£¬·¢ĻÖøĆŹŠŹ×ŅŖĪŪČ¾ĪļĪŖæÉĪüČėæÅĮ£ĪļPM2.5(Ö±¾¶Š”ÓŚµČÓŚ2.5umµÄŠüø”æÅĮ£Īļ)ĘäÖ÷ŅŖĄ“Ō“ĪŖČ¼Ćŗ”¢»ś¶Æ³µĪ²ĘųµČ£®Ņņ“Ė£¬¶ŌPM2.5”¢SO2”¢NOxµČ½ųŠŠŃŠ¾æ¾ßÓŠÖŲŅŖŅāŅ壮Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¶ŌPM2.5Ńł±¾ÓĆÕōĮóĖ®“¦ĄķÖĘ³É“ż²āŹŌŃł£®Čō²āµĆøĆŹŌŃłĖłŗ¬Ė®ČÜŠŌĪŽ»śĄė×ӵĻÆѧ×é·Ö¼°ĘäĘ½¾łÅضČČēĻĀ±ķ£ŗ
øł¾Ż±ķÖŠŹż¾ŻÅŠ¶ĻPM2.5µÄĖį¼īŠŌĪŖ________£¬ŹŌŃłµÄPHÖµ________
(2)ĪŖ¼õÉŁSO2µÄÅÅ·Å£¬³£²ÉČ”µÄ“ėŹ©ÓŠ£ŗ
¢Ł½«Ćŗ×Ŗ»ÆĪŖĒå½ąĘųĢåČ¼ĮĻ£®ŅŃÖŖ£ŗ
H2(g)£«1/2O2(g)£½H2O(g)””¦¤H£½£241.8 KJ/molC(s)£«1/2O2(g)£½CO(g)””¦¤H£½£110.5 KJ/mol
Š“³ö½¹ĢæÓėĖ®ÕōĘų·“Ó¦µÄČČ»Æѧ·½³ĢŹ½________£»
¢ŚĻ“µÓŗ¬SO2µÄŃĢĘų£¬ŅŌĻĀĪļÖŹæÉ×÷Ļ“µÓ¼ĮµÄŹĒ________£®
a£®Ca(OH)2
b£®Na2CO3
c£®CaCl2
d£®NaHSO3
(3)Ęū³µĪ²ĘųÖŠNOxŗĶCOµÄÉś³É¼°×Ŗ»ÆĪŖ£ŗ
¢ŁŅŃÖŖĘųø×ÖŠÉś³ÉNOµÄ·“Ó¦ĪŖ£ŗ
N2(g)£«O2(g)![]() 2NO(g)””¦¤H£¾0
2NO(g)””¦¤H£¾0
Čō1 molæÕĘųŗ¬ÓŠ0.8 mol””N2ŗĶ0.2 mol””O2£¬1300”ꏱŌŚĆܱÕČŻĘ÷ÄŚ·“Ó¦“ļµ½Ę½ŗā£®²āµĆNOĪŖ8”Į10£4 mol£®¼ĘĖćøĆĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżK£½________£®
Ęū³µĘō¶Æŗó£¬Ęųø×ĪĀ¶ČŌ½øߣ¬µ„Ī»Ź±¼äÄŚNOÅÅ·ÅĮæŌ½“ó£¬ŌŅņŹĒ________£®
¢ŚĘū³µČ¼ÓĶ²»ĶźČ«Č¼ÉÕŹ±²śÉśCO£¬ÓŠČĖÉčĻė°“ĻĀĮŠ·“Ó¦³żČ„CO£ŗ2CO(g)£½2C(s)£«O2(g)ŅŃÖŖøĆ·“Ó¦µÄ¦¤H£¾0£¬¼ņŹöøĆÉčĻėÄÜ·ńŹµĻÖµÄŅĄ¾Ż________£®
¢ŪÄæĒ°£¬ŌŚĘū³µĪ²ĘųĻµĶ³ÖŠ×°ÖĆ“ß»Æ×Ŗ»ÆĘ÷æɼõÉŁCOŗĶNOµÄĪŪČ¾£¬Ęä»Æѧ·“Ó¦·½³ĢŹ½ĪŖ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗɽ¶«Ź”ĘŚÖŠĢā ĢāŠĶ£ŗĢīæÕĢā
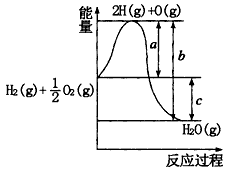
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com