
ŹµŃéŹŅÅäÖĘ500 mL 0.2 mol”¤L£1µÄNa2SO4ČÜŅŗ£¬ŹµŃé²Ł×÷²½ÖčÓŠ£ŗ
A£®ŌŚĢģĘ½ÉĻ³Ę³ö14.2 gĮņĖįÄĘ¹ĢĢ壬°ŃĖü·ÅŌŚÉÕ±ÖŠ£¬ÓĆŹŹĮæµÄÕōĮóĖ®Ź¹ĖüĶźČ«Čܽā²¢ĄäČ“ ÖĮŹŅĪĀ”£
B£®°ŃÖʵƵÄČÜŅŗŠ”ŠÄµŲ×ŖŅʵ½ČŻĮæĘæÖŠ”£
C£®¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮŅŗĆę¾ąæĢ¶ČĻß1”«2 cm“¦£¬øÄÓĆ½ŗĶ·µĪ¹ÜŠ”ŠÄµĪ¼ÓÕōĮóĖ®ÖĮČÜŅŗ°¼ŅŗĆę×īµĶ“¦ÓėæĢ¶ČĻßĻąĒŠ”£
D£®ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō2”«3“Ī£¬Ćæ“ĪĻ“µÓµÄŅŗĢ嶼Š”ŠÄ×¢ČėČŻĮæĘ棬²¢ĒįĒįÕńµ“”£
E£®½«ČŻĮæĘæĘæČūČū½ō£¬³ä·ÖŅ”ŌČŗó×°Ę攣
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©²Ł×÷²½ÖčµÄÕżČ·Ė³ŠņĪŖ(ĢīŠņŗÅ)____ _”£
£Ø2£©±¾ŹµŃéÓƵ½µÄ»ł±¾ŅĒĘ÷ŅŃÓŠÉÕ±”¢ĢģĘ½(“ųķĄĀė”¢Ä÷×Ó)”¢²£Į§°ō£¬»¹Č±ÉŁµÄŅĒĘ÷ŹĒ________”¢________”£
£Ø3£©ĻĀĮŠĒéæö»įŹ¹ĖłÅäČÜŅŗÅضČĘ«øߵďĒ(ĢīŠņŗÅ)______ __”£
A£®ČŻĮæĘæŹ¹ÓĆĒ°ÄŚ±ŚÕ“ÓŠĖ®Öé B£®Ć»½ųŠŠÉĻŹöµÄ²Ł×÷²½ÖčD
C£®¼ÓÕōĮóĖ®Ź±£¬²»É÷³¬¹żĮĖæĢ¶ČĻß D£®ķĄĀėÉĻÕ“ÓŠŌÓÖŹ
£Ø1£©A B D C E £Ø2£© 500 mL ČŻĮæĘæ µĪ¹Ü £Ø3£©D
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ÅäÖĘŅ»¶ØĢå»ż”¢Ņ»¶ØÅØ¶ČµÄČÜŅŗµÄ²½ÖčŹĒ¼ĘĖć”¢³ĘĮæ£Ø»ņĮæČ”£©”¢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ”£¹ŹÕżČ·Ė³ŠņŹĒA B D C E”££Ø2£©±¾ŹµŃéÓƵ½µÄ»ł±¾ŅĒĘ÷ŅŃÓŠÉÕ±”¢ĢģĘ½(“ųķĄĀė”¢Ä÷×Ó)”¢²£Į§°ō£¬»¹Č±ÉŁµÄŅĒĘ÷ŹĒ500 mL ČŻĮæĘæ µĪ¹Ü”££Ø3£©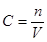 ”£ČōA£®ČŻĮæĘæŹ¹ÓĆĒ°ÄŚ±ŚÕ“ÓŠĖ®Ö飬Ōņ×īŗó¶ØČŻŹ±ÉŁ¼ÓŠ©Ė®£¬¶ŌČÜŅŗµÄÅضČĪŽÓ°Ļģ”£“ķĪó”£ČōB£®Ć»½ųŠŠÉĻŹöµÄ²Ł×÷²½ÖčD£¬ŌņÓÉÓŚČÜÖŹ¼õÉŁ£¬n¼õŠ”£¬ĖłŅŌÅضČĘ«Š””£“ķĪó”£ČōC£®¼ÓÕōĮóĖ®Ź±£¬²»É÷³¬¹żĮĖæĢ¶ČĻߣ¬ÓÉÓŚČÜŅŗµÄĢå»żĘ«“󣬵¼ÖĀÅضČĘ«µĶ”£“ķĪó”£D£®ČōķĄĀėÉĻÕ“ÓŠŌÓÖŹ£¬ŌņŅŌ“ĖķĄĀėĪŖ±ź×¼³ĘĮæµÄČÜÖŹµÄÖŹĮæĘ«“ó£¬nĘ«“ó£¬ĖłÅäČÜŅŗÅØ¶Č¾ĶĘ«“ó”£ÕżČ·”£
”£ČōA£®ČŻĮæĘæŹ¹ÓĆĒ°ÄŚ±ŚÕ“ÓŠĖ®Ö飬Ōņ×īŗó¶ØČŻŹ±ÉŁ¼ÓŠ©Ė®£¬¶ŌČÜŅŗµÄÅضČĪŽÓ°Ļģ”£“ķĪó”£ČōB£®Ć»½ųŠŠÉĻŹöµÄ²Ł×÷²½ÖčD£¬ŌņÓÉÓŚČÜÖŹ¼õÉŁ£¬n¼õŠ”£¬ĖłŅŌÅضČĘ«Š””£“ķĪó”£ČōC£®¼ÓÕōĮóĖ®Ź±£¬²»É÷³¬¹żĮĖæĢ¶ČĻߣ¬ÓÉÓŚČÜŅŗµÄĢå»żĘ«“󣬵¼ÖĀÅضČĘ«µĶ”£“ķĪó”£D£®ČōķĄĀėÉĻÕ“ÓŠŌÓÖŹ£¬ŌņŅŌ“ĖķĄĀėĪŖ±ź×¼³ĘĮæµÄČÜÖŹµÄÖŹĮæĘ«“ó£¬nĘ«“ó£¬ĖłÅäČÜŅŗÅØ¶Č¾ĶĘ«“ó”£ÕżČ·”£
æ¼µć£ŗæ¼²éĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗµÄÅäÖĘ²½Öč”¢4µÄŅĒĘ÷¼°Īó²ī·ÖĪöµČÖŖŹ¶”£


 æĘѧŹµŃé»ī¶Æ²įĻµĮŠ“š°ø
æĘѧŹµŃé»ī¶Æ²įĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)ŌŚŅ»¶ØĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬1Ģå»żX2(g)ŗĶ3Ģå»żY2(g)»ÆŗĻÉś³É2Ģå»żZ(g)£¬ŌņZĘųĢåµÄ»ÆѧŹ½ŹĒ________”£
(2)ŌŚĶ¬ĪĀ”¢Ķ¬Ń¹ĻĀ£¬ŹµŃé²āµĆCO”¢N2ŗĶO2ČżÖÖĘųĢåµÄ»ģŗĻĘųĢåµÄĆܶȏĒH2µÄ14.5±¶£¬ĘäÖŠO2µÄÖŹĮæ·ÖŹżĪŖ________”£ČōĘäÖŠCOŗĶN2µÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć1£¬Ōņ»ģŗĻĘųĢåÖŠŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ________”£
(3)ĻąĶ¬Ģõ¼žĻĀ£¬Ä³Cl2ÓėO2»ģŗĻĘųĢå100 mLĒ”ŗĆÓė150 mL H2»ÆŗĻÉś³ÉHClŗĶH2O£¬Ōņ»ģŗĻĘųĢåÖŠCl2ÓėO2µÄĢå»ż±ČĪŖ________£¬»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ________”£
(4)ĻÖÓŠm gijĘųĢ壬ĖüµÄĦ¶ūÖŹĮæĪŖM g”¤mol£1£¬Ōņ
¢ŁøĆĘųĢåČÜÓŚ1 LĖ®ÖŠ(²»æ¼ĀĒ·“Ó¦)£¬ĘäČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ________”£
¢ŚøĆĘųĢåČÜÓŚĖ®ŗóŠĪ³ÉV LČÜŅŗ£¬ĘäČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ______ mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°Ń2.0mol/LCuSO4ČÜŅŗŗĶ1.0mol/LH2SO4ČÜŅŗµČĢå»ż»ģŗĻ£Ø¼ŁÉč»ģŗĻŗóČÜŅŗµÄĢå»żµČÓŚĮ½ČÜŅŗµÄĢå»żÖ®ŗĶ£©”£
£Ø1£©ČÜŅŗÖŠH+µÄĪļÖŹµÄĮæÅضČĪŖ £¬SO42-µÄĪļÖŹµÄĮæÅضČĪŖ ”£
£Ø2£©Ļņ»ģŗĻČÜŅŗÖŠ¼ÓČė×ćĮæµÄĢś·Ū£¬¾×ć¹»³¤µÄŹ±¼äŗó£¬Ģś·ŪÓŠŹ£Óą”£“ĖŹ±£¬ČÜŅŗÖŠµÄFe2+ĪļÖŹµÄĮæÅضČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Č”20 mL NaOHČÜŅŗĘ½¾ł·Ö³ÉĮ½·Ż£¬·Ö±š·ÅČėA”¢BĮ½Ö§ŹŌ¹ÜÖŠ”£ĻņA”¢BÖŠĶØČė²»µČĮæµÄCO2£¬ŌŁ¼ĢŠųĻņĮ½ČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol/LµÄŃĪĖį£¬±ź×¼×“æöĻĀ²śÉśµÄCO2ĘųĢåĢå»żÓėĖł¼ÓµÄŃĪĖįČÜŅŗĢå»żÖ®¼äµÄ¹ŲĻµČēĻĀ±ķĖłŹ¾£ŗ
| ŃĪĖįĢå»ż£Øµ„Ī»£ŗmL£© | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| A²śÉśCO2µÄĢå»ż | 0 | 0 | 0 | 0 | 0 | 22.4 | 44.8 | 44.8 | 44.8 |
| B²śÉśCO2µÄĢå»ż | 0 | 0 | 22.4 | 44.8 | 67.2 | 89.6 | x | x | x |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
“Óŗ¬Ć¾”¢¼ŲŃĪŗžĖ®ÖŠÕō·¢×īŗóµĆµ½²śĪļÖŠŗ¬¹āĀ±ŹÆ(xKCl”¤yMgCl2”¤zH2O)£¬ĖüŹĒÖĘŌģ¼Ų·ŹŗĶĢįČ”½šŹōĆ¾µÄÖŲŅŖŌĮĻ£¬Ęä×é³ÉæÉĶعżĻĀĮŠŹµŃé²ā¶Ø£ŗ
¢Ł×¼Č·³ĘČ”5.550gѳʷČÜÓŚĖ®£¬Åä³É100.0mLČÜŅŗ”£
¢Ś½«ČÜŅŗ·Ö³É¶žµČ·Ż£¬ŌŚŅ»·ŻÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗÖĮ³ĮµķĶźČ«£¬¹żĀĖ”¢Ļ“µÓ”¢øÉ
ŌļÖĮŗćÖŲ£¬µĆµ½°×É«¹ĢĢå0.580g”£
¢ŪŌŚĮķŅ»·ŻČÜŅŗÖŠ¼ÓČė×ćĮæµÄĻõĖįĖį»ÆµÄAgNO3ČÜŅŗÖĮ³ĮµķĶźČ«£¬¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ
ÖĮŗćÖŲ£¬µĆµ½°×É«¹ĢĢå4.305g”£
£Ø1£©²½Öč¢ŚÖŠ¼ģŃé°×É«¹ĢĢåŅŃĻ“¾»µÄ·½·ØŹĒ£ŗ ”£
£Ø2£©ŅŃÖŖijĪĀ¶ČĻĀMg(OH)2µÄKsp£½6.4”Įl0?12£¬µ±ČÜŅŗÖŠc(Mg2+)”Ü1.0”Į10?5mol”¤L?1æÉŹÓĪŖ³ĮµķĶźČ«£¬Ōņ“ĖĪĀ¶ČĻĀÓ¦±£³ÖČÜŅŗÖŠc(OH£)”Ż mol”¤L?1”£
£Ø3£©Ķعż¼ĘĖćČ·¶ØѳʷµÄ×é³É£ØŠ“³ö¼ĘĖć¹ż³Ģ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĢģČ»¼īµÄ×é³ÉæÉŅŌÓĆ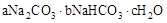 £Øa”¢b”¢cĪŖÕūŹż£©±ķŹ¾”£ĻÖÓŠA”¢BĮ½ÖÖ²»Ķ¬µÄĢģČ»¼īѳʷ£¬·Ö±š½ųŠŠČēĻĀŹµŃéŅŌČ·¶ØĘä»ÆѧŹ½”£
£Øa”¢b”¢cĪŖÕūŹż£©±ķŹ¾”£ĻÖÓŠA”¢BĮ½ÖÖ²»Ķ¬µÄĢģČ»¼īѳʷ£¬·Ö±š½ųŠŠČēĻĀŹµŃéŅŌČ·¶ØĘä»ÆѧŹ½”£
½«ÖŹĮæĪŖ31.0 gµÄĢģČ»¼īAÓŚ300”ę¼ÓČČ·Ö½āÖĮĶźČ«£Ø300”ꏱNa2CO3²»·Ö½ā£©£¬²śÉśCO2 2.24 L£Ø±ź×¼×“æö£©ŗĶĖ®5.4 g”£
£Ø1£©ĢģČ»¼īAµÄ»ÆѧŹ½ÖŠ£ŗ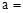 ””””””””””””””””
””””””””””””””””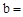 ””””””””””””””””
”””””””””””””””” ””””””””””””””””
””””””””””””””””
ŅŃÖŖ£ŗNa2CO3ÓėĻ”ŃĪĖįµÄ·“Ó¦·ÖĻĀĮŠĮ½²½½ųŠŠ£ŗ
Na2CO3+HCl NaCl+NaHCO3 NaHCO3+HCl
NaCl+NaHCO3 NaHCO3+HCl NaCl+CO2ӟ+H2O
NaCl+CO2ӟ+H2O
½«ÖŹĮæĪŖ12.45 gµÄĢģČ»¼īBČÜÓŚĖ®£¬ÖšµĪµĪ¼ÓijÅØ¶ČµÄĻ”ŃĪĖį£¬²śÉśĘųĢåµÄĢå»żÓė¼ÓČėŃĪ
ĖįµÄĢå»ż£Ø±ź×¼×“æö£©µÄ¹ŲĻµČēĻĀ±ķĖłŹ¾£ŗ
| ŃĪĖįĢå»ż£ØmL£© | 20 | 40 | 60 | 80 |
| ²śÉśĘųĢåĢå»ż£ØmL£© | 0 | 560 | 1680 | 2520 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³Ń§ÉśÓūÅäÖĘ6£®0 mol·L£1µÄH2SO4 1 000 mL£¬ŹµŃéŹŅÓŠČżÖÖ²»Ķ¬ÅØ¶ČµÄĮņĖį£ŗ¢Ł480 mL 0£®5 mol·L£1µÄĮņĖį£»¢Ś150 mL 25%µÄĮņĖį(¦Ń£½1£®18 g·mL£1)£»¢Ū×ćĮæµÄ18 mol·L£1µÄĮņĖį”£ÓŠČżÖÖ¹ęøńµÄČŻĮæĘæ£ŗ250 mL”¢500 mL”¢1 000 mL”£ĄĻŹ¦ŅŖĒó°Ń¢Ł¢ŚĮ½ÖÖĮņĖįČ«²æÓĆĶź£¬²»×ćµÄ²æ·ÖÓÉ¢ŪĄ“²¹³ä”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃéĖłÓĆ25%µÄĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ________mol·L£1(±£Įō1Ī»Š”Źż)”£
£Ø2£©ÅäÖĘøĆĮņĖįČÜŅŗӦєÓĆČŻĮæĘæµÄ¹ęøńĪŖ________mL”£
£Ø3£©ÅäÖĘŹ±£¬øĆĶ¬Ń§µÄ²Ł×÷Ė³ŠņČēĻĀ£¬Ēė½«²Ł×÷²½ÖčB”¢D²¹³äĶźÕū”£
A£®½«¢Ł¢ŚĮ½ČÜŅŗČ«²æŌŚÉÕ±ÖŠ»ģŗĻ¾łŌČ£»
B£®ÓĆĮæĶ²×¼Č·ĮæČ”ĖłŠčµÄ 18 mol·L£1µÄÅØĮņĖį mL£¬ŃŲ²£Į§°ōµ¹ČėÉĻŹö»ģŗĻŅŗÖŠ”£
²¢ÓĆ²£Į§°ō½Į°č£¬Ź¹Ęä»ģŗĻ¾łŌČ£»
C£®½«»ģŗĻ¾łŌȵÄĮņĖįŃŲ²£Į§°ō×¢ČėĖłŃ”µÄČŻĮæĘæÖŠ£»
D£®______________________________________£»
E£®Õńµ“£¬¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶ČĻß1”«2 cm“¦£»
F£®øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗµÄ°¼ŅŗĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ£»
G£®½«ČŻĮæĘæøĒ½ō£¬Õńµ“£¬Ņ”ŌČ”£
£Ø4£©Čē¹ūŹ”ĀŌ²Ł×÷D£¬¶ŌĖłÅäČÜŅŗÅضČÓŠŗĪÓ°Ļģ£æ________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
£Ø5£©½ųŠŠ²Ł×÷CĒ°»¹Šč×¢Ņā________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ņ»¶ØĮæµÄĒāĘųŌŚĀČĘųÖŠČ¼ÉÕ£¬ĖłµĆ»ģŗĻĪļÓĆ100ml 3.00mol/LµÄNaOHČÜŅŗĒ”ŗĆĶźČ«ĪüŹÕ£¬²āµĆČÜŅŗÖŠŗ¬ÓŠNaClOµÄĪļÖŹµÄĮæĪŖ0.05 mol”£
£Ø1£©ĖłµĆČÜŅŗÖŠCl-µÄĪļÖŹµÄĮæĪŖ mol”£
£Ø2£©²Ī¼Ó·“Ó¦µÄĒāĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ L”£(Š“³ö¼ĘĖć¹ż³Ģ£¬ĻĀĶ¬)
£Ø3£©ĖłÓĆĀČĘųŗĶ²Ī¼Ó·“Ó¦µÄĒāĘųµÄĪļÖŹµÄĮæÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚ»ØĘæÖŠ¼ÓČė”°ĻŹ»Ø±£ĻŹ¼Į”±£¬ÄÜŃÓ³¤ĻŹ»ØµÄŹŁĆü”£
£Ø1£©ĻÖÓŠŅ»ÖÖĪŽÉ«µÄĻŹ»ØÓŖŃųŅŗ£¬æÉÄÜÓÉĻõĖįøĘ”¢Ģ¼Ėį¼Ų”¢ĻõĖį¼Ų”¢ĀČ»Æ¼ŲÖŠµÄŅ»ÖÖ»ņ¼øÖÖĪļÖŹ×é³É£¬ĪŖĢ½¾æĘä³É·Ö£¬Ä³Ķ¬Ń§Éč¼Ę²¢Ķź³ÉĮĖČēĻĀĶ¼ĖłŹ¾µÄŹµŃ锣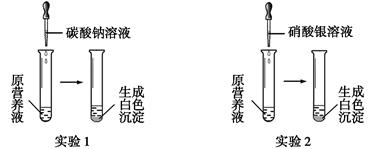
øł¾ŻŅŌÉĻŹµŃ飬ĒėÄćĢīæÕ”£
¢ŁÓÉŹµŃé1æÉČ·¶ØŌÓŖŃųŅŗÖŠŅ»¶ØƻӊµÄĪļÖŹŹĒ (Ģī»ÆѧŹ½)£¬Š“³öÉś³É°×É«³ĮµķµÄĄė×Ó·½³ĢŹ½ŹĒ ”£
¢ŚČō²āµĆŌÓŖŃųŅŗÖŠK+”¢Cl-µÄŹżÄæÖ®±ČĪŖ2”Ć1£¬ŌņŌÓŖŃųŅŗŹĒÓÉ ÖÖČÜÖŹÅäÖĘ³ÉµÄ”£
¢ŪijĶ¬Ń§ÓĆĀČ»ÆøĘ”¢ĻõĖį¼Ų”¢ĀČ»Æ¼ŲÅä³ÉµÄÓŖŃųŅŗÖŠK+”¢Cl-”¢NO3-µÄŹżÄæÖ®±ČĪŖ2”Ć5”Ć1£¬
ŌņĖłÓĆĻõĖį¼ŲŗĶĀČ»ÆøʵÄĪļÖŹµÄĮæÖ®±ČŹĒ ”£
£Ø2£©ĻĀ±ķŹĒ500mLij”°ĻŹ»Ø±£ĻŹ¼Į”±ÖŠŗ¬ÓŠµÄ³É·Ö£¬ŌĶĮŗó»Ų“šĻĀĮŠĪŹĢā”£
| ³É·Ö | ÖŹĮæ£Øg£© | Ħ¶ūÖŹĮæ£Øg ”¤mol-1£© |
| ÕįĢĒ | 68.4 | 342 |
| ĮņĖį¼Ų | 0.50 | 174 |
| °¢Ė¾Ę„ĮÖ | 0.35 | 180 |
| øßĆĢĖį¼Ų | 0.50 | 158 |
| ĻõĖįŅų | 0.04 | 170 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com