
£Ø14·Ö£©Q”¢W”¢X”¢Y”¢Z¾łĪŖŌŖĖŲÖÜĘŚ±ķÖŠĒ°ĖÄÖÜĘŚŌŖĖŲ£¬ĒŅĘäŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬QŌŖĖŲµÄŃōĄė×ÓŗĖĶāĪŽµē×Ó£¬WŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒĘä“ĪĶā²ćµē×ÓŹżµÄ2±¶£¬YŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£¬ZÄÜŠĪ³ÉŗģÉ«£Ø»ņשŗģÉ«£©µÄZ2OŗĶŗŚÉ«µÄZOĮ½ÖÖŃõ»ÆĪļ”£
¢ÅWĪ»ÓŚŌŖĖŲÖÜĘŚ±ķµŚ ÖÜĘŚµŚ ×唣YµÄµŚŅ»µēĄėÄÜ £ØĢī”°“óÓŚ”±»ņ”°Š”ÓŚ”±£©XµÄµŚŅ»µēĄėÄÜ”£
¢ĘXQ3·Ö×ÓÖŠµÄ»Æѧ¼üĄąŠĶĪŖ £ØĢī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©¹²¼Ū¼ü£¬æÕ¼äĄąŠĶĪŖ ”£Q”ŖX©pQ”ŖY©pQ”ŖWÖŠ¼ü³¤×ī¶ĢµÄŹĒ ”£
¢ĒZµÄ»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ £¬ZµÄµ„ÖŹÓėXµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĻ”ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
¢ČŅŃÖŖ£ŗ¢ŁWQ4(g) £«4XY2(g) ©4XY(g)£«WY2 (g)£«2Q2Y(g) ”÷H©£574KJ”¤mol-1
¢ŚWQ4(g) £«4XY(g) ©2X2 (g) £«WY2 (g) £«2Q2Y(g) ”÷H©£1160KJ”¤mol-1
ŌņÓÉWQ4»¹ŌXY2Éś³ÉX2µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø1£©¶ž”¢¢ōA£¬Š”ÓŚ£»£Ø2£©¼«ŠŌ”¢Čż½Ē׶ŠĪ”¢H-O£»
£Ø3£©1s22s23s23p63d104s1”¢3Cu+8H++2NO3-=3Cu2++2NO”ü+2H2O£»
£Ø4£©CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g) ¦¤H=£867KJ/mol£»
½āĪöŹŌĢā·ÖĪö£ŗøł¾ŻĢāŅāæÉÖŖÕā¼øÖÖŌŖĖŲ·Ö±šŹĒ£ŗQŹĒH£»WŹĒC£»XŹĒN£»YŹĒO£»ZŹĒCu”£¢ÅCŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÖŠĪ»ÓŚµŚ¶žÖÜĘŚµŚ¢ōA£»ÓÉÓŚNŌŖĖŲµÄŌ×ÓµÄ×īĶā²ćµÄµē×Ó“¦ÓŚøĆ¹ģµĄµÄ°ė³äĀśµÄĪȶØדĢ¬£¬Ź§Č„µē×Ó½ĻÄŃ£¬ĖłŅŌNµÄµŚŅ»µēĄėÄÜ“óÓŚOµÄµŚŅ»µēĄėÄÜ”£¢ĘŌŚNH3·Ö×ÓÖŠµÄ»Æѧ¼üĄąŠĶĪŖ¼īŠŌ¹²¼Ū¼ü£¬øĆ·Ö×ÓµÄæÕ¼äĄąŠĶĪŖČż½Ē׶ŠĪ£»ÓÉÓŚC”¢N”¢OČżÖÖŌŖĖŲµÄŌ×Ó°ė¾¶Öš½„¼õŠ”£¬ĖłŅŌC”ŖH£»N”ŖH£»O”ŖHµÄ¼ü³¤Ō½Ą“Ō½¶Ģ£¬¼üÄܾĶŌ½Ą“Ō½“ó£¬Ņņ“Ė¼ü³¤×ī¶ĢµÄO”ŖH¼ü£»¢ĒŌ×Ó“¦ÓŚČ«³äĀś”¢°ė³äĀś»ņČ«æÕŹ±ŹĒĪČ¶ØµÄדĢ¬£¬Ņņ“Ė29ŗÅŌŖĖŲCuµÄ»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ1s22s23s23p63d104s1; CuµÄµ„ÖŹÓėNµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļHNO3µÄĻ”ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ3Cu+8H++2NO3-=3Cu2++2NO”ü+2H2O£»£Ø4£©(¢Ł+¢Ś)”Ā2ÕūĄķæɵĆCH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g) ¦¤H=£867KJ/mol£»
æ¼µć£ŗæ¼²éŌŖĖŲµÄĶʶĻ”¢ĪļÖŹ·Ö×ÓÖ®¼ä»Æѧ¼ü”¢·Ö×ÓµÄæռ乹ŠĶ”¢Ō×ӵĵē×ÓÅŲ¼Ź½”¢ŌŖĖŲµÄµēĄėÄÜµÄ±Č½Ļ”¢Ąė×Ó·½³ĢŹ½”¢ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“µÄÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
a”¢b”¢c”¢d”¢eŹĒµŚČżÖÜĘŚµÄĪåÖÖŌŖĖŲ£¬aŗĶbµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĻŌ¼īŠŌ£¬ĒŅ¼īŠŌb£¾a£¬cŗĶdµÄĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌc£¾d£¬eµÄµ„ÖŹ¼ČÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬Ņ²ÄÜÓėĻ”ĮņĖį·“Ó¦£¬²¢ĒŅ¶¼²śÉśĒāĘų£¬ŌņĖüĆĒµÄŌ×ÓŠņŹżÓÉŠ”µ½“óµÄĖ³ŠņŹĒ
| A£®a”¢b”¢e”¢d”¢c | B£®b”¢a”¢c”¢e”¢d |
| C£®b”¢a”¢e”¢d”¢c | D£®b”¢a”¢d”¢c”¢e |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ÄæĒ°£¬Ņ½ĮĘÉĻŹ¹ÓĆ·ÅÉäŠŌŗĖĖŲ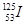 ÖĪĮĘÖ×Įö£¬øĆŗĖĖŲŌ×ÓŗĖÄŚµÄÖŠ×ÓŹżÓėµē×ÓŹżÖ®²īŹĒ
ÖĪĮĘÖ×Įö£¬øĆŗĖĖŲŌ×ÓŗĖÄŚµÄÖŠ×ÓŹżÓėµē×ÓŹżÖ®²īŹĒ
| A£® 125 | B£®72 | C£®19 | D£®53 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(µŚ1Š”Ģā1·Ö,ĘäÓąĆææÕ2·Ö,¹²11 ·Ö)
¶ĢÖÜĘŚŌŖĖŲA”¢B”¢C”¢DÖŠ£¬0.5 mol AŌŖĖŲµÄĄė×ӵƵ½6.02”Į1023øöµē×Ó±»»¹ŌĪŖÖŠŠŌŌ×Ó£¬0.4 g AµÄŃõ»ÆĪļĒ”ŗĆÓė100 mL 0.2 mol/LµÄŃĪĖįĶźČ«·“Ó¦£¬AŌ×ÓŗĖÄŚÖŹ×ÓŹżÄæÓėÖŠ×ÓŹżÄæĻąµČ£¬BŌŖĖŲŹĒµŚ¶žÖÜĘŚÖŠŌ×Ó°ė¾¶×īŠ”µÄŌŖĖŲ£¬C2£±ČAŌŖĖŲµÄĄė×Ó¶ą1øöµē×Ó²ć£¬DŌŖĖŲµÄŌ×ÓŗĖĶāL²ć±ČK²ć¶ą2øöµē×Ó”£
£Ø1£©BŌŖĖŲµÄĆū³ĘŹĒ ”£
£Ø2£©»³öDŗĶC2£µÄ½į¹¹Ź¾ŅāĶ¼ ”£______________________”£
£Ø3£©½«BŌŖĖŲŠĪ³ÉµÄµ„ÖŹĶØČėĖ®ÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________£¬ B”¢C”¢DČżÖÖŌŖĖŲŠĪ³ÉµÄĒā»ÆĪļÖŠĪČ¶ØŠŌ×īĒæµÄŹĒ_______£ØĢīŠ“»ÆѧŹ½£¬ĻĀĶ¬£©£¬×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļĖįŠŌ×īĒæµÄŹĒ_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ÓŠA”¢B”¢C”¢D”¢EĪåÖÖŌ×ÓŠņŹżŠ”ÓŚ18µÄŌŖĖŲ£¬ĘäĢŲÕ÷ŠÅĻ¢ČēĻĀ£ŗ
| ŌŖĖŲ±ąŗÅ | ĢŲÕ÷ŠÅĻ¢ |
| A | ĘäÖŠŅ»ÖÖŌ×ÓŗĖÄŚÖ»ÓŠÖŹ×Óƻӊ֊×Ó |
| B | ĘäŌ×ÓµÄL²ćµē×ÓŹżŹĒK²ćµÄ3±¶ |
| C | ĘäŃōĄė×ÓÓėBµÄŅõĄė×Ó¾ßÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹£¬ ĒŅŗĖµēŗÉŹżÓėBĻą²ī3 |
| D | ĘäŌ×ÓµÄ×īĶā²ćµē×ÓŹżµČÓŚµē×Ó²ćŹż£¬ĒŅŹĒµŲæĒ ÖŠŗ¬Įæ½Ļ¶ąµÄŌŖĖŲÖ®Ņ» |
| E | µ„ÖŹĪŖ»ĘĀĢÉ«ĘųĢ壬æÉÓĆÓŚÖĘŌģĘÆ°×·Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö, Õė¶Ō±ķÖŠµÄ¢Ł”«¢įÖÖŌŖĖŲ,ĢīŠ“ĻĀĮŠæÕ°×:
| Ö÷×å ÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0×å |
| 2 | | | | ¢Ł | ¢Ś | ¢Ū | | |
| 3 | ¢Ü | | ¢Ż | | | ¢Ž | ¢ß | ¢ą |
| 4 | ¢į | | | | | | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©A”¢B”¢C”¢D ĖÄÖÖÖ÷×åŌŖĖŲŌ×ÓŠņŹż¾łŌŚ20ŅŌÄŚ£¬AŌŖĖŲĖł“¦µÄÖÜĘŚŹż”¢Ö÷×åŠņŹż”¢Ō×ÓŠņŹż¾łĻąµČ£¬BµÄŌ×Ó°ė¾¶ŹĒĘäĖłŌŚÖ÷×åÖŠ×īŠ”µÄ£¬BµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄ»ÆѧŹ½ĪŖHBO3 £¬CŌŖĖŲŌ×ÓµÄ×īøßÕż¼ŪĪŖ+6¼Ū£¬CµÄŅõĄė×ÓÓėDµÄŃōĄė×Ó¾ßÓŠĻąĶ¬µÄµē×ÓÅŲ¼£¬DæÉÓėCŠĪ³É»ÆŗĻĪļD2C”£
£Ø1£©DŌŖĖŲµÄĆū³Ę_______£»BŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£ŗµŚ_______ÖÜĘŚµŚ_______×å
£Ø2£©A”¢BŠĪ³ÉµÄ»ÆŗĻĪļŅŌ_______¹²¼Ū¼üĻą½įŗĻ£ØĢī¼«ŠŌ»ņ·Ē¼«ŠŌ£©
£Ø3£©ŅŌCµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ×÷ĪŖµē½āÖŹČÜŅŗ£¬×é³ÉĒāŃõČ¼ĮĻµē³Ų£¬·ÅµēŹ±Ōņøŗ¼«ĒųČÜŅŗµÄpH_______£ØĢīŌö“󔢼õŠ””¢²»±ä£©
£Ø4£©ŅŌDµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ×÷ĪŖµē½āÖŹČÜŅŗ£¬×é³ÉĒāŃõČ¼ĮĻµē³Ų£¬ŌņÕż¼«µÄµē¼«·“Ó¦Ź½ĪŖ_____________________
£Ø5£©A”¢B”¢C”¢DĖÄÖÖŌŖĖŲ¼ņµ„Ąė×ӵĥė×Ó°ė¾¶Óɓ󵽊”µÄĖ³ŠņŹĒ£ØÓĆĄė×Ó·ūŗűķŹ¾£© ____________________________
£Ø6£©CµÄÖŹ×ÓŹżŗĶÖŠ×ÓŹżĻąµČ£¬ŌņC µÄŌ×Ó×é³É·ūŗÅĪŖ_______ĖüŠĪ³ÉµÄĒā»ÆĪļµÄ»¹ŌŠŌ_______ĀČ»ÆĒā£ØĢīĒæÓŚ»ņČõÓŚ£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
”¢ĻĀĶ¼ŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬ĘäÖŠĆæøöŹż×Ö±ąŗÅ“ś±ķ¶ŌÓ¦µÄŅ»ÖÖŌŖĖŲ”£
| ¢Ł | | | ||||||
| | | | | ¢Ś | ¢Ū | ¢Ü | | |
| ¢Ż | | ¢Ž | ¢ß | | | ¢ą | ¢į | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©”¾Ń”×öĢā”æ±¾Ģā°üĄØA”¢BĮ½Š”Ģā£¬ĒėŃ”¶ØĘäÖŠŅ»Š”Ģā£¬²¢ŌŚĻąÓ¦µÄ“šĢāĒųÓņÄŚ×÷“š”£Čō¶ą×ö£¬Ōņ°“AŠ”ĢāĘĄ·Ö”£
A£®[ĪļÖŹ½į¹¹ÓėŠŌÖŹ]
ŌŖĖŲX»łĢ¬Ō×ÓµÄpµē×Ó±Čsµē×ÓÉŁ1øö”£ŌŖĖŲY»łĢ¬Ō×ÓµÄ2pµē×ÓŹĒ2sµē×ÓµÄ2±¶”£ŌŖĖŲZµÄŅ»ÖÖµ„ÖŹĪŖ×ŌČ»½ēÓ²¶Č×ī“óµÄĪļÖŹ”£ŌŖĖŲMĪ»ÓŚµŚĖÄÖÜĘŚ£¬Ę仳Ģ¬Ō×ÓŹ§Č„3øöµē×Óŗó3d¹ģµĄ°ėĀś”£
£Ø1£©X”¢Y”¢ZµÄ»łĢ¬Ō×Ó£¬µŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒ ”£
£Ø2£©XµÄĒā»ÆĪļŅ×ÓŚŅŗ»Æ£¬ĘäŌŅņŹĒ ”£
£Ø3£©XÓėMŠĪ³ÉµÄŅ»ÖÖ»ÆŗĻĪļ¾§°ū½į¹¹ČēĶ¼ĖłŹ¾”£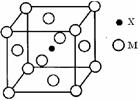
¢ŁĆæøö¾§°ūÖŠMĄė×ӵďżÄæĪŖ £»
¢ŚøĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©YæÉŠĪ³Éµ„ÖŹY3
¢ŁY3·Ö×ÓµÄæռ乹ŠĶĪŖ £ØÓĆĪÄ×ÖĆčŹö£©”£
¢ŚŠ“³öŅ»ÖÖÓėY3»„ĪŖµČµē×ÓĢåµÄ·Ö×ӵĻÆѧŹ½£ŗ ”£
£Ø5£©Y”¢ZµÄ»ÆŗĻĪļZYæÉÓėMµÄµ„ÖŹÉś³ÉÅäŗĻĪļM(ZY)5£¬øĆÅäŗĻĪļÖŠ¦Ņ¼üÓė¦Š¼üµÄøöŹż±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com