
ĻĀĶ¼ŹĒµŖŌŖĖŲµÄ¼øÖÖ¼ŪĢ¬ÓėĪļÖŹĄą±šµÄ¶ŌÓ¦¹ŲĻµ£ŗ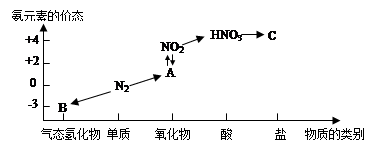
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öN2µÄŅ»ÖÖÓĆĶ¾ ”£
£Ø2£©“ÓNŌŖĖŲ»ÆŗĻ¼Ū·ÖĪö£¬N2¾ßÓŠŃõ»ÆŠŌŗĶ»¹ŌŠŌ”£ø÷¾ŁŅ»ĄżĖµĆ÷£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©
“š£ŗŃõ»ÆŠŌ ”£
»¹ŌŠŌ ”£
£Ø3£©HNO3ÓėÉĻĶ¼ÖŠµÄĪļÖŹC³£ÓĆÓŚ¼ģŃéCl-µÄ“ęŌŚ£¬ŌņCµÄ»ÆѧŹ½ĪŖ________”£
£Ø4£©ŹµŃéŹŅÖĘČ”ĪļÖŹBµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©NO2ÓėĖ®·“Ӧɜ³ÉĪļÖŹAµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø6£©ÅØĻõĖįÓėľĢæŌŚ¼ÓČČĢõ¼žĻĀ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø1£©×ö±£»¤Ęų”¢±£“ęĮøŹ³”¢ÖĘ°±ĘųµČ”£
£Ø2£©N2+3H2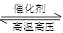 2NH3£¬ N2+O2
2NH3£¬ N2+O2 2NO»ņN2+3Mg
2NO»ņN2+3Mg Mg3N2µČ
Mg3N2µČ
£Ø3£©AgNO3
£Ø4£©Ca(OH)2+2NH4Cl CaCl2+2NH3”ü+2H2O
CaCl2+2NH3ӟ+2H2O
£Ø5£©3NO2+H2O£½2HNO3+NO
£Ø6£©4HNO3(ÅØ)+C CO2”ü+4NO2”ü+2H2O
CO2ӟ+4NO2ӟ+2H2O
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ÓÉÓŚµŖĘų»ÆѧŠŌÖŹ²»»īĘĆ£¬ĖłŅŌ×ö±£»¤Ęų”¢±£“ęĮøŹ³”¢ÖĘ°±ĘųµČ”££Ø2£©N2µĆµ½µē×Ó£¬»ÆŗĻ¼Ū½µµĶ£¬±ķĻÖŃõ»ÆŠŌ£ŗN2+3H2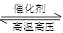 2NH3£»N2Ź§Č„µē×Ó£¬»ÆŗĻ¼ŪÉżøߣ¬±ķĻÖ»¹ŌŠŌ£ŗN2+O2
2NH3£»N2Ź§Č„µē×Ó£¬»ÆŗĻ¼ŪÉżøߣ¬±ķĻÖ»¹ŌŠŌ£ŗN2+O2 2NO»ņN2+3Mg
2NO»ņN2+3Mg Mg3N2”££Ø3£©Cl-µÄ¼ģŃé·½·ØŹĒĻņ“ż²āČÜŅŗÖŠ¼ÓČėĻõĖįĖį»Æ£¬ŌŁµĪ¼ÓAgNO3ČÜŅŗ£¬Čō²śÉś²»ŹĒ³Įµķ£¬¾ĶÖ¤Ć÷ČÜŅŗÖŠŗ¬ÓŠCl-”£Ņņ“ĖCĪŖAgNO3”££Ø4£©ŌŚŹµŃéŹŅÖŠŹĒÓĆļ§ŃĪÓė¼ī¹²ČČĄ“ÖĘČ”°±ĘųµÄ”£ÖĘČ”ĪļÖŹ°±ĘųµÄ»Æѧ·½³ĢŹ½ĪŖCa(OH)2+2NH4Cl
Mg3N2”££Ø3£©Cl-µÄ¼ģŃé·½·ØŹĒĻņ“ż²āČÜŅŗÖŠ¼ÓČėĻõĖįĖį»Æ£¬ŌŁµĪ¼ÓAgNO3ČÜŅŗ£¬Čō²śÉś²»ŹĒ³Įµķ£¬¾ĶÖ¤Ć÷ČÜŅŗÖŠŗ¬ÓŠCl-”£Ņņ“ĖCĪŖAgNO3”££Ø4£©ŌŚŹµŃéŹŅÖŠŹĒÓĆļ§ŃĪÓė¼ī¹²ČČĄ“ÖĘČ”°±ĘųµÄ”£ÖĘČ”ĪļÖŹ°±ĘųµÄ»Æѧ·½³ĢŹ½ĪŖCa(OH)2+2NH4Cl CaCl2+2NH3”ü+2H2O”££Ø5£©NO2ÓėĖ®·“Ӧɜ³ÉĪļÖŹNOµÄ»Æѧ·½³ĢŹ½ĪŖ3NO2+H2O£½2HNO3+NO”££Ø6£©ÅØĻõĖįÓŠĒæŃõ»ÆŠŌ£¬ŌŚ¼ÓČČĢõ¼žĻĀÓėľĢæ·¢Éś·“Ó¦²śÉśCO2”¢NO2”¢H2O”£·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ4HNO3(ÅØ)+C
CaCl2+2NH3”ü+2H2O”££Ø5£©NO2ÓėĖ®·“Ӧɜ³ÉĪļÖŹNOµÄ»Æѧ·½³ĢŹ½ĪŖ3NO2+H2O£½2HNO3+NO”££Ø6£©ÅØĻõĖįÓŠĒæŃõ»ÆŠŌ£¬ŌŚ¼ÓČČĢõ¼žĻĀÓėľĢæ·¢Éś·“Ó¦²śÉśCO2”¢NO2”¢H2O”£·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ4HNO3(ÅØ)+C CO2”ü+4NO2”ü+2H2O”£
CO2ӟ+4NO2ӟ+2H2Oӣ
æ¼µć£ŗ漲鰱ĘųµÄŹµŃéŹŅÖĘ·Ø”¢Cl-µÄ¼ģŃ锢¼°µŖŌŖĖŲµÄµ„ÖŹ¼°»ÆŗĻĪļµÄŠŌÖŹµÄ»Æѧ·½³ĢŹ½±ķŹ¾µÄÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀČŹĒÖŲŅŖµÄ·Ē½šŹōŌŖĖŲ”£
30.NaClŗĶÅØĮņĖįĪ¢ČČÖĘČ”ĀČ»ÆĒāµÄ»Æѧ·½³ĢŹ½ĪŖ £»
æÉÓĆ ŹŌÖ½·ÅŌŚĘææŚŅŌ¼ģŃéĀČ»ÆĒāĘųĢåŹĒ·ń¼ÆĀś”£
31.ŠĀÖĘĀČĖ®ÖŠŗ¬ÓŠµÄ·Ö×ÓÓŠ£ŗCl2”¢H2OŗĶ £»¹āÕÕŠĀÖĘĀČĖ®µÄ»Æѧ·½³ĢŹ½ĪŖ £»¹¤ŅµŹĒÓƵē½āŹ³ŃĪĖ®ÖĘČ”ĀČĘų£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗ
2H++2e”śH2”ü,ŌņŃō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
32.ŅŃÖŖ»¹ŌŠŌSO32-£¾I-£¾Br-.ĻņNaBr”¢NaI”¢Na2SO3»ģŗĻČÜŅŗÖŠ£¬ĶØČė”Ŗ¶ØĮæĀČĘųŗ󣬽«ČÜŅŗÕōøɲ¢³ä·Ö×ĘÉÕ£¬µĆµ½¹ĢĢåŹ£ÓąĪļÖŹµÄ×é³ÉæÉÄÜŹĒ______£ØŃ”Ģī±ąŗÅ£©”£
a. NaCl Na2SO4 b. NaCl NaBr Na2SO4
c. NaCl Na2SO4 I2 d. NaCl NaI Na2SO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÓĆĶÖʱøCuSO4ČÜŅŗÓŠ¶ąÖÖ·½°ø£¬Ä³ŹµŃ銔×éøų³öĮĖŅŌĻĀČżÖÖ·½°ø£ŗ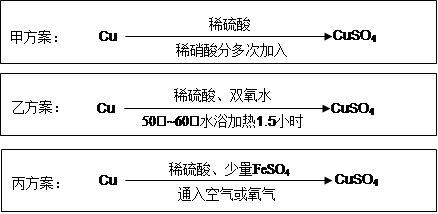
Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©¼×·½°ø£ŗ
¢ŁŠ“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ £»
¢ŚĪŖĮĖ½ŚŌ¼ŌĮĻ£¬ĮņĖįŗĶĻõĖįµÄĪļÖŹµÄĮæÖ®±Č×ī¼ŃĪŖ£¬n(H2SO4)£ŗn(HNO3)= ”£
£Ø2£©ŅŅ·½°ø£ŗ½«6.4gĶĖæ·Åµ½90mL 1.5mol”¤L-1µÄĻ”ĮņĖįÖŠ£¬æŲĪĀŌŚ50”ę”£¼ÓČė40mL 10%µÄH2O2£¬·“Ó¦0.5Š”Ź±£¬ÉżĪĀµ½60”ę£¬³ÖŠų·“Ó¦1Š”Ź±ŗ󣬾Ņ»ĻµĮŠ²Ł×÷£¬µĆCuSO4”¤5H2O 20.0g”¾ŅŃÖŖÓŠ¹ŲĦ¶ūÖŹ Įæ£ŗM(Cu)=64g/mol£¬ M(CuSO4”¤5H2O) =250g/mol”攣
¢Ł·“Ó¦Ź±ĪĀ¶ČæŲÖĘŌŚ50”ę~60”ę£¬²»ŅĖ¹żøßµÄŌŅņŹĒ £»
¢Ś±¾ŹµŃéCuSO4”¤5H2OµÄ²śĀŹĪŖ ”£
£Ø3£©±ū·½°ø£ŗ½«æÕĘų»ņŃõĘųÖ±½ÓĶØČėµ½Ķ·ŪÓėĻ”ĮņĖįµÄ»ģŗĻĪļÖŠ£¬·¢ĻÖŌŚ³£ĪĀĻĀ¼øŗõ²»·“Ó¦”£Ļņ·“Ó¦ŅŗÖŠ¼ÓÉŁĮæFeSO4£¬¼“·¢Éś·“Ó¦£¬Éś³ÉĮņĖįĶ”£·“Ó¦ĶźČ«ŗ󣬼ÓĪļÖŹAµ÷½ŚpHÖĮ4 £¬Č»ŗó¹żĀĖ”¢ÅØĖõ”¢½į¾§”£
¢ŁĪļÖŹAæÉŃ”ÓĆŅŌĻĀµÄ £ØĢīŠņŗÅ£©£»
| A£®CaO | B£®NaOH | C£®CuCO3 | D£®Cu2(OH)2CO3 E£®Fe2(SO4)3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŹĒÉśĆüÖ®Ō“”£ŅūÓĆĖ®Ļū¶¾×÷ĪŖæŲÖĘĖ®ÖŹµÄŅ»øöÖŲŅŖ»·½Ś£¬ŅŗĀČĻū¶¾ŹĒ×īŌēµÄŅūÓĆĖ®Ļū¶¾·½·Ø”£½üÄźĄ“æĘѧ¼ŅĢį³ö£¬ĀČĘųÄÜÓėĖ®ÖŠµÄÓŠ»śĪļ·¢Éś·“Ó¦£¬Éś³ÉµÄÓŠ»śĀČ»ÆĪļæÉÄܶŌČĖĢåÓŠŗ¦”£¶žŃõ»ÆĀČ£ØClO2£©ŹĒŅ»ÖÖŌŚĖ®“¦ĄķµČ·½ĆęÓŠ¹ć·ŗÓ¦ÓƵÄøߊ§°²Č«Ļū¶¾¼Į”£ÓėCl2Ļą±Č£¬ClO2²»µ«¾ßÓŠøüĻŌÖųµŲɱ¾śÄÜĮ¦£¬¶ųĒŅ²»»į²śÉś¶ŌČĖĢåÓŠĒ±ŌŚĪ£ŗ¦µÄÓŠ»śĀČ“śĪļ”£
£Ø1£©ĀČĘųČÜÓŚĖ®ÄÜɱ¾śĻū¶¾µÄŌŅņŹĒ ”£
£Ø2£©ŌŚClO2µÄÖʱø·½·ØÖŠ£¬ÓŠĻĀĮŠĮ½ÖÖÖʱø·½·Ø£ŗ
·½·ØŅ»£ŗNaClO3£«4HCl=2ClO2”ü£«Cl2”ü£«2NaCl£«2H2O
·½·Ø¶ž£ŗC6H12O6+24NaClO3+12H2SO4=24ClO2”ü+6CO2”ü+18H2O+12Na2SO4
ÓĆ·½·Ø¶žÖʱøµÄClO2øüŹŹŗĻÓĆÓŚŅūÓĆĖ®µÄĻū¶¾£¬ĘäÖ÷ŅŖŌŅņŹĒ ”£
£Ø3£©ÓĆClO2“¦Ąķ¹żµÄŅūÓĆĖ®£ØpHĪŖ5£®5~6£®5£©³£ŗ¬ÓŠŅ»¶ØĮæ¶ŌČĖĢå²»ĄūµÄŃĒĀČĖįøłĄė×Ó£ØClO2££©£®2001ÄźĪŅ¹śĪĄÉś²æ¹ę¶Ø£¬ŅūÓĆĖ®ClO2£µÄŗ¬ĮæÓ¦²»³¬¹ż0£®2 mg”¤L£1”£
ŅūÓĆĖ®ÖŠClO2”¢ClO2£µÄŗ¬ĮææÉÓĆĮ¬ŠųµāĮæ·Ø½ųŠŠ²ā¶Ø”£ClO2±»I£»¹ŌĪŖClO2£”¢Cl£µÄ×Ŗ»ÆĀŹÓėČÜŅŗpHµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£µ±pH”Ü2£®0Ź±£¬ClO2£Ņ²Äܱ»I£ĶźČ«»¹Ō³ÉCl£”£·“Ӧɜ³ÉµÄI2ÓƱź×¼Na2S2O3ČÜŅŗµĪ¶Ø£ŗ2Na2S2O3£«I2=Na2S4O6£«2NaI
¢ŁĒėŠ“³öpH”Ü2£®0Ź±£¬ClO2£ÓėI£·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
¢ŚÅäÖĘNa2S2O3±ź×¼ČÜŅŗŹ±£¬Ź¹ÓƵÄŅĒĘ÷³żĢģĘ½”¢Ņ©³×”¢²£Į§°ō”¢ÉÕ±”¢Įæ¼ņĶā£¬»¹ŠčŅŖĻĀĶ¼ÖŠµÄ £ØĢī×ÖÄø“śŗÅ£©”£




a b c d e
¢ŪĒėĶź³ÉĻąÓ¦µÄŹµŃé²½Öč£ŗ
²½Öč1£ŗ×¼Č·ĮæČ”VmLĖ®Ńł¼ÓČėµ½×¶ŠĪĘæÖŠ”£
²½Öč2£ŗµ÷½ŚĖ®ŃłµÄpHĪŖ7£®0~8£®0
²½Öč3£ŗ¼ÓČė×ćĮæµÄKI¾§Ģ唣
²½Öč4£ŗ¼ÓÉŁĮæµķ·ŪČÜŅŗ£¬ÓĆc mol”¤L-1Na2S2O3ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄNa2S2O3ČÜŅŗV1mL”£
²½Öč5£ŗµ÷½ŚČÜŅŗµÄpH”Ü2£®0”£
²½Öč6£»ŌŁÓĆc mol”¤L-1Na2S2O3ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄNa2S2O3ČÜŅŗV2mL”£
¢ÜµĪ¶ØÖÕµćµÄĻÖĻóŹĒ ”£
¢Żøł¾ŻÉĻŹö·ÖĪöŹż¾Ż£¬²āµĆøĆŅżÓĆĖ®ŃłÖŠµÄClO2£µÄÅضČĪŖ mg”¤L£1£ØÓĆŗ¬×ÖÄøµÄ“śŹżŹ½±ķŹ¾£©”£
£Ø4£©ÅŠ¶ĻĻĀĮŠ²Ł×÷¶ŌClO2£µÄÅØ¶Č²ā¶Ø½į¹ūµÄÓ°Ļģ£ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©
¢Ł ČōŌŚÅäÖʱź×¼ČÜŅŗ¹ż³ĢÖŠ£¬ÉÕ±ÖŠµÄNa2S2O3ČÜŅŗÓŠÉŁĮ潦³ö£¬Ź¹²ā¶Ø½į¹ū ”£
¢Ś ČōŌŚµĪ¶ØÖÕµć¶ĮČ”µĪ¶Ø¹ÜæĢ¶ČŹ±£¬ø©ŹÓ±ź×¼ŅŗŅŗĆę£¬Ź¹²ā¶Ø½į¹ū ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ÖŠA”«J·Ö±š“ś±ķĻą¹Ų·“Ó¦µÄŅ»ÖÖĪļÖŹ”£ŅŃÖŖA·Ö½āµĆµ½µČĪļÖŹµÄĮæµÄB”¢C”¢D£¬Ķ¼ÖŠÓŠ²æ·ÖÉś³ÉĪļĪ“±ź³ö”£
ĒėĢīŠ“ŅŌĻĀæÕ°×£ŗ
(1)AµÄ»ÆѧŹ½ ”£
(2)¶Ø³ö·“Ó¦¢Ł¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł £¬
¢Ś ”£
(3)Š“³ö·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½£ŗ ”£
(4)JÓėF·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
(5)ŌŚ·“Ó¦¢ÜÖŠ£¬µ±Éś³É±źæöĻĀ2.24 L GŹ±£¬×ŖŅʵē×ÓŹżĪŖ mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÅØĮņĖį¾ßÓŠŅŌĻĀA”«FµÄŠŌÖŹ£ŗAĖįŠŌ£»Bøß·ŠµćÄѻӷ¢£»CĪüĖ®ŠŌ£»DĶŃĖ®ŠŌ£»EĒæŃõ»ÆŠŌ£»FČÜÓŚĖ®·Å³ö“óĮæČČ
(1)ÅØĮņĖįÓėĶ¹²ČČ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£ŹµŃéÖŠĶłĶłÓŠ“óĮæĄ¶É«¹ĢĢåĪö³ö£¬æɼūÅØĮņĖįŌŚøĆŹµŃéÖŠ±ķĻֵĊŌÖŹÓŠ ”£(ÅØĮņĖįŠŌÖŹÓĆ”°A”±”¢”°B”±”¢”°C”±”¢”°D”±”¢”°E”±”¢”°F”±ĢīæÕ£¬ĻĀĶ¬)
(2)ŹµŃéÖ¤Ć÷Ķ²»ÄÜŌŚµĶĪĀĻĀÓėO2·“Ó¦£¬Ņ²²»ÄÜÓėĻ”ĮņĖį¹²ČČ·¢Éś·“Ó¦£¬µ«¹¤ŅµÉĻČ“ŹĒ½«·ĻĶŠ¼µ¹ČėČȵÄĻ”ĮņĖįÖŠ²¢ĶØČėæÕĘųĄ“ÖʱøCuSO4ČÜŅŗ”£ĶŠ¼ŌŚ“ĖדĢ¬ĻĀ±»ČܽāµÄ»Æѧ·½³ĢŹ½ĪŖ ”£ĮņĖįŌŚøĆ·“Ó¦ÖŠ±ķĻֵĊŌÖŹŹĒ ”£
(3)ŌŚ¹żŃõ»ÆĒāÓėĻ”ĮņĖįµÄ»ģŗĻČÜŅŗÖŠ¼ÓČėĶʬ£¬³£ĪĀĻĀ¾ĶÉś³ÉĄ¶É«ČÜŅŗ”£Š“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£Óė(2)ÖŠ·“Ó¦±Č½Ļ£¬·“Ó¦Ģõ¼ž²»Ķ¬µÄŌŅņŹĒ ”£
(4)ĻņÕįĢĒ¾§ĢåÖŠµĪ2”«3µĪĖ®£¬ŌŁµĪČėŹŹĮæµÄÅØĮņĖį”£·¢ĻÖ¼ÓĖ®“¦Į¢¼“±äŗŚ£¬ŗŚÉ«Ēų²»¶ĻĄ©“ó£¬×īŗó±ä³ÉŅ»æéŹčĖɵĽ¹Ģ棬²¢°éÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå²śÉś”£Š“³ö²śÉśÓŠ“Ģ¼¤ĘųĪ¶ĘųĢåµÄ»Æѧ·½³ĢŹ½£ŗ ”£øĆŹµŃéÖŠÅØĮņĖį±ķĻֵĊŌÖŹÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶žŃõ»ÆĀČÅŻĢŚĘ¬£¬ÓŠŠ§³É·Ö£ØClO2£©ŹĒŅ»ÖÖøߊ§”¢°²Č«µÄɱ¾ś”¢Ļū¶¾¼Į”£
·½·ØŅ»£ŗĀČ»ÆÄʵē½ā·ØŹĒŅ»ÖÖæÉææµÄ¹¤ŅµÉś²śClO2ĘųĢåµÄ·½·Ø”£øĆ·Ø¹¤ŅÕŌĄķČēĶ¼”£Ęä¹ż³ĢŹĒ½«Ź³ŃĪĖ®ŌŚĢŲ¶ØĢõ¼žĻĀµē½āµĆµ½µÄĀČĖįÄĘ£ØNaClO3£©ÓėŃĪĖį·“Ӧɜ³ÉClO2”£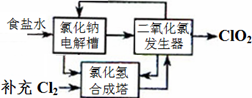
£Ø1£©¹¤ŅÕÖŠæÉĄūÓƵĵ„ÖŹÓŠ__________£ØĢī»ÆѧŹ½£©£¬·¢ÉśĘ÷ÖŠÉś³ÉClO2µÄ»Æѧ·½³ĢŹ½ĪŖ_____________”£
£Ø2£©“Ė·ØµÄȱµćÖ÷ŅŖŹĒ______________________________________”£
·½·Ø¶ž£ŗ×ī½ü£¬æĘѧ¼ŅÓÖŃŠ¾æ³öĮĖŅ»ÖÖŠĀµÄÖʱø·½·Ø£¬ĻĖĪ¬ĖŲ»¹Ō·ØÖĘClO2£¬ĘäŌĄķŹĒ£ŗĻĖĪ¬ĖŲĖ®½āµĆµ½µÄ×īÖÕ²śĪļXÓėNaClO3·“Ӧɜ³ÉClO2”£
£Ø3£©ÅäĘ½·½³ĢŹ½£ŗ ”õ £ØX£© +”õNaClO3+”õH2SO4”ś”õClO2”ü+”õCO2”ü+”õH2O+”õ______
Čō·“Ó¦ÖŠ²śÉś4.48L£ØÕŪĖć³É±ź×¼×“æöĻĀ£©ĘųĢ壬µē×Ó×ŖŅĘ________ øö”£
£Ø4£©ClO2ŗĶCl2¾łÄܽ«µē¶Ę·ĻĖ®ÖŠµÄCN”ŖŃõ»ÆĪŖĪŽ¶¾µÄĪļÖŹ£¬×ŌÉķ±»»¹ŌĪŖCl”Ŗ”£“¦Ąķŗ¬CN”ŖĻąĶ¬ĮæµÄµē¶Ę·ĻĖ®£¬ĖłŠčCl2µÄĪļÖŹµÄĮæŹĒClO2µÄ_______±¶”£
·½·ØČż£ŗŹµŃéŹŅ³£ÓĆĀČĖįÄĘ(NaClO3)ŗĶŃĒĮņĖįÄĘ(Na2SO3)ÓĆĮņĖįĖį»Æ£¬¼ÓČČÖʱø¶žŃõ»ÆĀČ£¬»Æѧ·“Ó¦·½³ĢŹ½ĪŖ£ŗ2NaClO3+Na2SO3£«H2SO4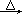 2ClO2”ü+2Na2SO4£«H2O
2ClO2”ü+2Na2SO4£«H2O
£Ø5£©·“Ó¦ÖŠµÄNa2SO3ČÜŅŗÖŠ“ęŌŚČēĻĀĘ½ŗā£ŗH2O H++OH-ŗĶ ________________£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©.
H++OH-ŗĶ ________________£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©.
³£ĪĀĻĀ£¬0.1mol/LøĆČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”ÅÅĮŠ__________________£ØÓĆĄė×Ó·ūŗűķŹ¾£©
£Ø6£©³£ĪĀĻĀ£¬ŅŃÖŖNaHSO3ČÜŅŗ³ŹĖįŠŌ£¬ŌŚNa2SO3ČÜŅŗÖŠµĪ¼ÓĻ”ŃĪĖįÖĮÖŠŠŌŹ±£¬ČÜÖŹµÄÖ÷ŅŖ³É·ÖÓŠ________________”££ØÓĆ»ÆѧŹ½±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŌĮņĢśæó£ØÖ÷ŅŖ³É·ÖĪŖFeS2£©ĪŖŌĮĻÖʱøĀČ»ÆĢś¾§Ģå£ØFeCl3”¤6H2O£©µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ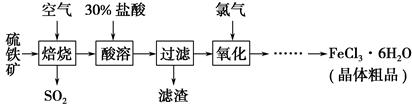
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬SO2×Ŗ»ÆĪŖSO3µÄ·“Ó¦ĪŖ2SO2£Øg£©£«O2£Øg£©??2SO3£Øg£©£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK£½________£»¹żĮæµÄSO2ÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
_____________________________________________________________ӣ
£Ø2£©ĖįČܼ°ŗóŠų¹ż³ĢÖŠ¾łŠč±£³ÖŃĪĖį¹żĮ棬ĘäÄæµÄŹĒ________”¢________”£
£Ø3£©ĶØĀČĘųŃõ»ÆŹ±£¬·¢ÉśµÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________£»øĆ¹ż³Ģ²śÉśµÄĪ²ĘųæÉÓĆ¼īČÜŅŗĪüŹÕ£¬Ī²ĘųÖŠĪŪČ¾æÕĘųµÄĘųĢåĪŖ________£ØŠ“»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°×ÓńµÄ»ÆѧŹ½æÉÓĆCaxMgySipO22(OH)2±ķŹ¾£ØŅ²æÉÓĆCa”¢Mg”¢Si”¢HµÄŃõ»ÆĪļ±ķŹ¾£©”£
£Ø1£©Č”8.l0g°×Óń·ŪÄ©×ĘÉÕÖĮŗćÖŲ£¬¹ĢĢå¼õÉŁĮĖ0.18g£¬Ōņ°×ÓńµÄĦ¶ūÖŹĮæĪŖ______”£
£Ø2£©ĮķČ”4.05g°×Óń·ŪÄ©¼ÓČėl mol/LµÄŃĪĖįl00mLÖŠ³ä·ÖČܽā£¬×īÖÕµĆ²»ČÜŃõ»ÆĪļ2. 40g.¹żĀĖ£¬½«ĀĖŅŗŗĶĻ“µÓŅŗŗĻ²¢ŗóĶłĘäÖŠ¼ÓČė×ćĮæµÄĢśŠ¼£¬µĆµ½ĘųĢå336mL(±ź×¼×“æöĻĀ).Ōņ
¢Łp=_________£»
¢Ś°×ÓńµÄ»ÆѧŹ½£ØÓĆŃõ»ÆĪļµÄŠĪŹ½£©±ķŹ¾ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com