
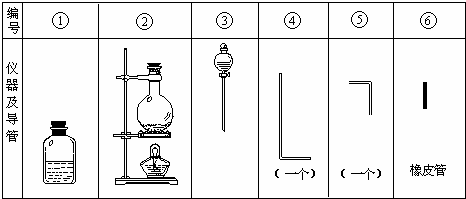
���д�ʵ�飬���õ���������������ͼ.

����Ҫ����д���пհף�
(1)��װ����������ʱ��Ӧѡ�õ�����������(��дͼ�б��)��________.
(2)ʵ������У���������������ҵ�˳�����������ĸ����������ܵı��������________.
(3)����������Ƥ����Ӧ��________���ף�ԭ����___________________.
(4)ʵ��ʱ���������г��۲쵽������_______________________����.
(5)ʵ������Լ10��H2O2��Һ100mL����������30��(�ܶȽ���Ϊ1g��cm-3)H2O2�����ƣ���������Ʒ�����__________________________________________________________.
(6)ʵ��ʱ��������ClO-��H2O2��Ӧ�����ӷ���ʽ��________________________.
| (1)�ۢڢ�
(2)�ڢݢޢܢ� (3) 2��ʹƿ����ѹǿ���(д�������������������ͨ�ķ���������Ҳ���) (4)ð���� (5)����Ͳȡ33mL(��34mL)30��H2O2��Һ�����ձ��У��ټ���67mL(��66mL)ˮ(���ˮϡ����100mL)��������� (6)
|
| ����ѧ��ѧ��ѧ�У�������ʵ�����Ʒ��ǹ�Һ���ȵķ�Ӧ�������װ������ʱӦѡ�ڡ��ۼ�����.����ѡ�ܻ��Ǣ�Ҫ��������ʵ�飬ע��ͼ����ȷָ���ܺ͢ݶ�����һ������������ʵ��װ���У���Ҫ������У���������װ������ʱֻ��ѡ���ܢ�.�ڻش�����(2)ʱ��Ҫ������������Ҫ����Ƥ�����ӣ������ȷ�Ļش��Ǣڢݢޢܢ�.����(3)Ҫע�����������μӻ����ɵķ�Ӧ��ʵ��װ�ã�һ����Ҫ���Dz�©�������������(��������ķ�����ֱ������μӻ�ѧ��Ӧ֮ǰ��װ�ò��ֶ���Ӧ©��)����һ����ҲҪ����װ�õ����(ָ���巢����ѧ��Ӧ֮���װ�ò���)Ҫ�������ͨ��ʹ����������Ӧ�������������ų�(���ſ�)������������Щ���岻���ų���ʹ��װ����ѹ����������ᷢ����ը.��ˢ�����Ƥ����Ӧ��2��(��2������)�ף���ʹƿ����ѹǿ���.�����֪��H2O2����ԭ��ʱ����������ΪO2.���ܸ��ܵ�O2ת�����ͨO2�����������Թ�(��ɫ)��ʽ�ų�����O2�������壬���ʵ��ʱ�������г��۲쵽�����ɹ۲쵽ð���ݵ�����.����(5)����Һ��������ѧ��ѧʵ�������������֮һ.Ҫע����ĿҪ��������Ʒ�������Ҫ˵����ȡŨ��Һ��������ʲô������ȡ������ʲô�����У��������ˮϡ�ͺͽ�����ȵ����Ƶ�ȫ����.����(6) H2O2��ClO-�ķ�Ӧ�в�����O2���ɴ˿��ƶϳ�ǿ�������H2O2��ClO-��Ӧ��H2O2�ǻ�ԭ����ClO-���������������ֻ����Cl-.����ClO-��H2O2��Ӧ�����ӷ���ʽ�ǣ�
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
������������Ҫ������������ԭ��������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȡ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ�˹�������ĺ�������̽���˹�����������ʡ�

��.�ⶨ��������ĺ���
��1��ȡ10.00 mL�ܶ�Ϊ�� g/mL�Ĺ���������Һϡ����250mL����ȡϡ�ͺ�Ĺ���������Һ25.00mL����ƿ�У�����ϡ�����ữ��������ˮϡ�ͣ��������������ø�����ر���Һ�ζ�����������д���ζ������з�����Ӧ�����ӷ���ʽ�� _____________________________________________________
��2���ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й����������������Ϊ____________��
��.̽���������������
��1��H2O2��ͭ���й�̽��ʵ�飺��ͭ˿�����������ữ��H2O2��Һ�У�ͭ��Ѹ����������Һ������ͬʱ�����������壬������������ʹ���ľ����ȼ����Ӧ�����ӷ���ʽΪ��_________________________��
�ڽ�ͭ˿����H2O2��Һ�У�û�����ݲ�����������Һʱ���۲쵽ͭ˿��������������壬��������ʹ���ľ����ȼ���ڸñ仯��ͭ˿�����������__________��д������������һ����ķ�Ӧ�Ļ�ѧ����ʽ__________________________��
��2���������õ���ͨ���Ũ��NaOH��H2O2�Ļ��Һ�У��ڵ��ܿ�����Һ�ĽӴ�������˸�ĺ����֣�������Ϊͨ������Һ�в�����ClO����H2O2��ԭ���������ҷ�Ӧ,���������ϸߵ������ӣ�������ת��Ϊ��ͨ�����ӣ�������������Ժ��ų���ClO����H2O2��Ӧ�����ӷ���ʽ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
�������õ���ͨ���Ũ��NaOH��H2O2�Ļ��Һ�У��ڵ��ܿ�����Һ�ĽӴ�������˸�ĺ����֡�������Ϊͨ������Һ�в�����ClO����H2O2��ԭ���������ҷ�Ӧ�����������ϸߵ������ӣ�Ȼ������ת��Ϊ��ͨ�����ӣ�����������Ժ��ų�����ʵ�����õ�������������ͼ��
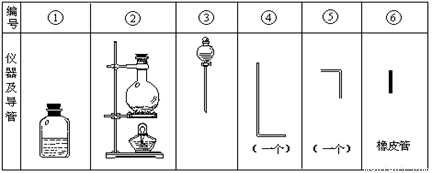
����Ҫ����д���пհף�
��1����װ��������װ��ʱ��Ӧѡ�õ�����������Ϊ (��дͼ�б��)��
��2����ʵ�����ʱ����������������ҵ�˳�����������ĸ����������ܵı������Ϊ ��
��3�������ٵ���Ƥ��������Ӧ��2����ԭ���� ��
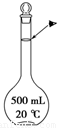
��4����ʵ��������10mol��L��1��NaOH��Һ500mL���õ�����������������ƽ���ձ��⣬�����õ��������� (����������) ������ʱ������ͼ����������ҺŨ�� (�ƫ�ߡ���ƫ�͡�)��
��5��ʵ��ʱ��������ClO����H2O2��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com