
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���֪��101kPaʱ��16.0gN2H4����������ȫȼ�����ɵ������ų�����312kJ��25��ʱ����д����ʾN2H4ȼ���ȵ��Ȼ�ѧ����ʽ ��
��2������----����ȼ�ϵ����20%~30%��KOH��ҺΪ�������Һ��
�����ĵ缫��Ӧʽ�� ��
�����ĵ缫��Ӧʽ�� ��
��3����ͼ��һ���绯ѧ����ʾ��ͼ��
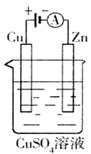
��пƬ�Ϸ����ĵ缫��Ӧ�� ��
�ڼ���ʹ���¡�����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128g������һ����ȼ�ϵ�����������ı��״���µĿ��� L����������������������Ϊ20%��
��1��N2H4��l��+O2(g) == N2(g)+2H2O(g) ;��H=-624 kJ/mol
��2��2O2+4H2O+8e==8OH- CH4+10OH-��8e==CO32-+7H2O
��3��Cu2++2e=Cu 112L
��1������������ʽ���Ӻ������ɵ�����ȫȼ�յ��Ȼ�ѧ����ʽN2H4��g��+O2��g��=N2��g��+2H2O��g����H=��534kJ/mol����2����طŵ�ʱ������������ԭ��Ӧ����O2����ԭ���缫��ӦʽΪO2+2H2O+4e-=4OH-������ʱ��������ˮ���ɣ���KOH��ҺŨ�ȼ�С��pH��С��


 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
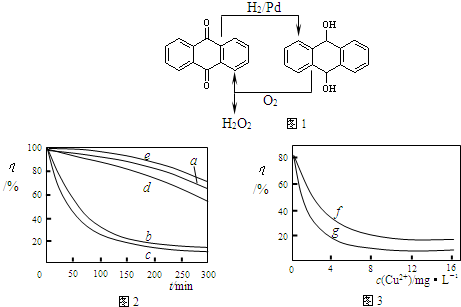
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com