
×ī½ü£¬ĪŅ¹śĄūÓĆÉś²śĮ×ļ§ÅŷŵķĻŌüĮ׏ÆøąÖĘČ”ĮņĖį²¢ĮŖ²śĖ®ÄąµÄ¼¼ŹõŃŠ¾æ»ńµĆ³É¹¦”£¾ßĢåÉś²śĮ÷³ĢČēĶ¼£ŗ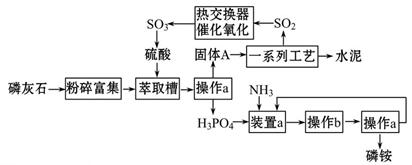
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²Ł×÷aµÄĆū³ĘŹĒ___________£¬ŹµŃéŹŅÖŠ½ųŠŠ“Ė²Ł×÷µÄ·Ē²£Į§ŅĒĘ÷»ņÓĆĘ·ÓŠ________________£»ŌŚŹµŃéŹŅÖŠ²Ł×÷bµÄĆū³ĘŹĒ______________________”£
£Ø2£©×°ÖĆaÖŠÉś³ÉĮ½ÖÖĖįŹ½ŃĪ£¬ĖüĆĒµÄ»ÆѧŹ½·Ö±šŹĒ_______________”£
£Ø3£©ŅĄĢāŅā²Ā²ā¹ĢĢåAÖŠŅ»¶Øŗ¬ÓŠµÄĪļÖŹµÄ»ÆѧŹ½ŹĒ____________________(½į¾§Ė®²æ·Ö²»Š“)”£
£Ø4£©ČČ½»»»Ę÷ŹĒŹµĻÖĄäČČ½»»»µÄ×°ÖĆ”£»ÆѧŹµŃéÖŠŅ²¾³£ĄūÓĆČČ½»»»Ą“ŹµĻÖijÖÖŹµŃéÄæµÄ£¬Ęų”¢ŅŗČČ½»»»Ź±Ķس£Ź¹ÓƵÄŅĒĘ÷ŹĒ________________________”£
£Ø5£©ÖĘĮņĖįĖł²śÉśµÄĪ²Ęų³żĮĖŗ¬ÓŠN2”¢O2Ķā£¬»¹ŗ¬ÓŠSO2£¬Ī¢ĮæµÄSO3ŗĶĖįĪķ”£ÄÜÓĆÓŚ²ā¶ØĮņĖįĪ²ĘųÖŠSO2ŗ¬ĮæµÄŹĒ___________________”£
| A£®NaOHČÜŅŗ”¢·ÓĢŖŹŌŅŗ | B£®KMnO4ČÜŅŗ”¢Ļ”ĮņĖį |
| C£®µāĖ®”¢µķ·ŪČÜŅŗ | D£®°±Ė®”¢·ÓĢŖŹŌŅŗ |
£Ø1£©¹żĀĖ Ģś¼ÜĢØ(ŗ¬ĢśČ¦)”¢ĀĖÖ½ Õō·¢ÅØĖõ”¢ĄäČ“½į¾§
£Ø2£©NH4H2PO4”¢(NH4)2HPO4
£Ø3£©CaSO4 £Ø4£©ĄäÄż¹Ü £Ø5£©B”¢C
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¾¹ż²Ł×÷aµĆµ½¹ĢĢåAŗĶH3PO4ŅŗĢ壬·ÖĄė¹ĢĢåŗĶŅŗĢåµÄ·½·ØŹĒ¹żĀĖ£¬ĖłŅŌ²Ł×÷aµÄĆū³ĘŹĒ¹żĀĖ£»¹żĀĖŠčŅŖŹ¢·ÅŅ©Ę·µÄÉÕ±”¢¹żĀĖµÄĀ©¶·”¢ŅżĮ÷×÷ÓĆµÄ²£Į§°ō”¢¹Ģ¶ØĀ©¶·µÄĢś¼ÜĢØ£Øŗ¬ĢśČ¦£©”¢ĀĖÖ½µČ£¬ĖłŅŌ·Ē²£Į§ŅĒĘ÷»ņÓĆĘ·ÓŠ£ŗĢś¼ÜĢØ(ŗ¬ĢśČ¦)”¢ĀĖÖ½£»“ÓČÜŅŗÖŠĪö³ö¾§ĢåµÄ·½·ØŹĒ£ŗ½«ČÜŅŗÕō·¢ÅØĖõ”¢ĄäČ“½į¾§æɵĆĻąÓ¦¾§Ģ壬ĖłŅŌŌŚŹµŃéŹŅÖŠ²Ł×÷bµÄĆū³ĘŹĒ£ŗÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”£
£Ø2£©×°ÖĆaÖŠĮ×ĖįÓė°±Ęų·¢Éś·“Ó¦£¬Į×ĖįŹĒČżŌŖĖį£¬Óė°±Ęų·“Ó¦æÉŅŌÉś³É£ØNH4£©3PO4”¢£ØNH4£©2HPO4”¢NH4H2PO4ČżÖÖŃĪ£¬ĘäÖŠ£ØNH4£©2HPO4”¢NH4H2PO4Į½ÖÖĖįŹ½ŃĪ”£
£Ø3£©ÓÉŠÅĻ¢æÉÖŖÉś²śĮ×ļ§ÅŷŵķĻŌüĮ׏ÆøąÖĘČ”ĮņĖį£¬Į×»ŅŹÆµÄÖ÷ŅŖ³É·ÖŹĒCa3£ØPO4£©2£¬ŌŚŻĶČ”²ŪÖŠÓėĮņĖį·¢Éś·“Ó¦£¬ÓŠĮ×ĖįÉś³É£¬½įŗĻĮ÷³ĢÖŠĮņĖįÖʱøĮ÷³Ģ£¬¹ĢĢåA¾¹żŅ»ĻµĮŠ·“Ó¦æÉÖĘČ”H2SO4£¬¹Ź¹ĢĢåAÓ¦ĪŖCaSO4”£
£Ø4£©ÄÜŹµĻÖĘų”¢ŅŗĢåČČ½»»»µÄ×°ÖĆŹĒĄäÄż¹Ü£¬ĻĀæŚ½ųĖ®ÉĻæŚ“¦Ė£¬ĘųĢåŗĶĖ®Į÷·½ĻņĻą·“£¬³ä·Ö½ųŠŠČČ½»»»£¬ĖłŅŌøĆŅĒĘ÷ĪŖĄäÄż¹Ü”£
£Ø5£©A”¢NaOHČÜŅŗÓėSO2”¢Ī¢ĮæµÄSO3ŗĶĖįĪķ·“Ó¦£¬²āĮæµÄSO2ŗ¬ĮæĘ«øߣ¬“ķĪó£»B”¢ĮņĖįĪ²ĘųÖŠÖ»ÓŠSO2Äܱ»ĖįŠŌKMnO4ČÜŅŗŃõ»Æ£¬ČÜŅŗŃÕÉ«ÓÉ×ĻŗģÉ«±äĪŖĪŽÉ«£¬øł¾ŻKMnO4ČÜŅŗµÄĢå»ż½įŗĻ·½³ĢŹ½¼ĘĖćSO2µÄŗ¬Įæ£¬ÕżČ·£»C”¢ĮņĖįĪ²ĘųÖŠÖ»ÓŠSO2Äܱ»µāĖ®Ńõ»ÆSO2£¬ČÜŅŗŃÕÉ«ÓÉĄ¶É«±äĪŖĪŽÉ«£¬øł¾ŻµāĖ®ČÜŅŗµÄĢå»ż½įŗĻ·½³ĢŹ½¼ĘĖćSO2µÄŗ¬Įæ£¬ÕżČ·£»D”¢°±Ė®NaOHČÜŅŗÓėSO2”¢Ī¢ĮæµÄSO3ŗĶĖįĪķ·“Ó¦£¬²āĮæµÄSO2ŗ¬ĮæĘ«øߣ¬“ķĪó”£
æ¼µć£ŗ±¾Ģāæ¼²é»Æѧɜ²śĮ÷³ĢµÄ·ÖĪö”¢ŹµŃ黳±¾²Ł×÷ŗĶ»ł±¾ŅĒĘ÷”¢²śĪļµÄÅŠ¶Ļ¼°²āĮ攣



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹµŃé²Ł×÷ÄÜŹ¹ŹµŃé½į¹ūĘ«µĶµÄŹĒ
| A£®ÓĆÕōĮóĖ®ČóŹŖµÄpHŹŌÖ½Ėł²ā¶ØµÄijĖįČÜŅŗµÄpH |
| B£®ÓĆČŻĮæĘæÅäÖĘČÜŅŗ£¬¶ØČŻŗóŅ”ŌČŅŗĆęĻĀ½µ£¬ŌŁ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻßĖłÅäÖʵÄČÜŅŗÅØ¶Č |
| C£®ÓĆŃöŹÓĮæĶ²æĢ¶ČĮæČ”µÄŅ»¶ØĮæÅØĮņĖįĖłÅäÖʵÄ0.1mol”¤L-1H2SO4ČÜŅŗµÄÅØ¶Č |
| D£®ÓĆ“ż²āŅŗČóĻ“µÄ׶ŠĪĘæ½ųŠŠÖŠŗĶµĪ¶ØĖł²ā¶ØµÄ“ż²āŅŗÅØ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µŖ»ÆĀĮ(AlN)ŹĒŅ»ÖÖŠĀŠĶĪŽ»ś²ÄĮĻ£¬¹ć·ŗÓ¦ÓĆÓŚ¼Æ³ÉµēĀ·Éś²śĮģÓņ”£ČōijµŖ»ÆĀĮÖŠŗ¬ÓŠĢ¼»ņŃõ»ÆĀĮÖŠµÄŅ»ÖÖ£¬ĻÖÓĆĶ¼IÖŠµÄŅ»Š©×°ÖĆĄ“½ųŠŠ¼ģŃ飬Ź¹µŖ»ÆĀĮѳʷŗĶNaOHČÜŅŗ·“Ó¦£ŗAlN£«NaOH£«H2O=NaAlO2£«NH3”ü£¬øł¾Ż·“Ó¦ÖŠĖłÉś³É°±ĘųµÄĢå»żĄ“²ā¶Øѳʷ֊µÄµŖ»ÆĀĮµÄÖŹĮæ·ÖŹż£¬²¢øł¾ŻŹµŃéĻÖĻóĄ“Č·¶ØŌÓÖŹµÄ³É·Ö(ŹµŃéÖŠµ¼¹ÜĢå»żŗöĀŌ²»¼Ę)”£
(1)ŹµŃéÓŠ¹Ų²Ł×÷ĪŖ£ŗa.ĶłÉÕĘæÖŠ·ÅČėŹŹĮæµÄAlNѳʷ£ŗb.“Ó·ÖŅŗĀ©¶·ĶłÉÕĘæÖŠ¼ÓČė¹żĮæµÄÅØNaOHČÜŅŗ£ŗc.¼ģŃé×°ÖƵÄĘųĆÜŠŌ£»d.²ā¶ØŹÕ¼Æµ½Ė®µÄĢå»ż”£
ÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ£ŗ ”£
(2)±¾ŹµŃéÖŠ(Ķ¼¢ń)¼ģ²é×°ÖĆĘųĆÜŠŌµÄ·½·ØŹĒ£ŗ ”£
(3)¹ćæŚĘæÖŠµÄŹŌ¼ĮXæÉŃ”ÓĆ (ĢīŃ”ĻīĒ°µÄ±źŗÅ)”£
| A£®ĘūÓĶ | B£®¾Ę¾« | C£®Ö²ĪļÓĶ | D£®CCl4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ba2£«ŹĒŅ»ÖÖÖŲ½šŹōĄė×Ó£¬ÓŠŅ»»·¾³¼ą²āŠ”×éÓūĄūÓĆNa2S2O3”¢KI”¢K2Cr2O7µČŹŌ¼Į²ā¶Øij¹¤³§·ĻĖ®ÖŠBa2£«µÄĪļÖŹµÄĮæÅØ¶Č”£
(1)ŌŚČŻĮæĘæµÄŹ¹ÓĆ·½·ØÖŠ£¬ĻĀĮŠ²Ł×÷²»ÕżČ·µÄŹĒ(Ģī×ÖÄø)________”£
| A£®Ź¹ÓĆČŻĮæĘæĒ°¼ģ²éĖüŹĒ·ńĀ©Ė® |
| B£®ČŻĮæĘæÓĆĖ®Ļ“¾»ŗó£¬ŌŁÓĆ“żÅäČÜŅŗČóĻ“ |
| C£®ÅäÖĘČÜŅŗŹ±£¬Čē¹ūŹŌŃłŹĒ¹ĢĢ壬°Ń³ĘŗƵďŌŃłÓĆÖ½ĢõŠ”ŠÄµ¹ČėČŻĮæĘæÖŠ£¬»ŗĀż¼ÓĖ®ÖĮ½Ó½ü±źĻß1”«2 cm“¦£¬ÓƵĪ¹ÜÖšµĪµĪ¼ÓÕōĮóĖ®ÖĮ±źĻß |
| D£®ÅäÖĘČÜŅŗŹ±£¬ČōŹŌŃłŹĒŅŗĢ壬ÓĆĮæĶ²ĮæČ”ŹŌŃłŗóÖ±½Óµ¹ČėČŻĮæĘæÖŠ£¬»ŗĀż¼ÓĖ®ÖĮ½Ó½ü±źĻß1”«2 cm“¦£¬ÓƵĪ¹ÜÖšµĪµĪ¼ÓÕōĮóĖ®ÖĮ±źĻß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij»ÆѧŹµŃ銔×éĢ½¾æŹŠŹŪŹ³Óưד×ÖŠ“×ĖįµÄµÄ×¼Č·ÅØ¶Č£¬Č”25.00mLÄ³Ę·ÅĘŹ³Óưד×ӌ׶ŠĪĘæÖŠ£¬ŌŚŹµŃéŹŅÓĆÅضČĪŖc””mol/LµÄ±ź×¼NaOHČÜŅŗ¶ŌĘä½ųŠŠµĪ¶Ø”£
£Ø1£©ČēĶ¼±ķŹ¾50mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬ČōAÓėCæĢ¶Č¼äĻą²īl mL£¬A“¦µÄæĢ¶ČĪŖ25£¬µĪ¶Ø¹ÜÖŠŅŗĆę¶ĮŹżÓ¦ĪŖ””””””””””mL”£
£Ø2£©ĪŖĮĖ¼õŠ”ŹµŃéĪó²ī£¬øĆĶ¬Ń§Ņ»¹²½ųŠŠĮĖČż“ĪŹµŃ飬¼ŁÉčĆæ“ĪĖłČ”°×“×Ģå»ż¾łĪŖVmL£¬NaOH±ź×¼ŅŗÅضČĪŖc mo1/L£¬Čż“ĪŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 26.02 | 25.35 | 25.30 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖÖ÷µÄæĘѧ£¬»ÆѧŹµŃéŹĒѧĻ°Ģ½¾æĪļÖŹŠŌÖŹµÄ»ł±¾·½·ØÖ®Ņ»”£
£Ø1£©ĻĀĮŠÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ__________£ØĢīŠ“ŠņŗÅ£©
a£®Ź¹ÓĆĶŠÅĢĢģĘ½µÄµŚŅ»²½²Ł×÷ŹĒ½«ÓĪĀėŅĘÖĮ±ź³ßĮćæĢ¶Č“¦
b£®¹żĀĖ²Ł×÷¹ż³ĢÖŠ£¬ĪŖ¼Óæģ¹żĀĖĖŁ¶ČæÉÓĆ²£Į§°ō¶ŌĀ©¶·ÖŠµÄČÜŅŗ½ųŠŠ½Į°č
c£®ÓĆÅØĮņĖįÅäÖĘĻ”ČÜŅŗŹ±£¬ŌŚĮæĶ²ÖŠŗāĻ”ŗóŅŖĄäČ“ÖĮŹŅĪĀŌŁ×ŖŅʵ½ČŻĮæĘæÖŠ
d£®ÓĆČŻĮæĘæÅäÖĘČÜŅŗŹ±£¬¶ØČŻŗóŅ”ŌČŅŗĆęĻĀ½µ£¬ŌŁ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß“¦£¬ĖłµĆČÜŅŗÅضČĘ«µĶ
£Ø2£©ĻÖÓŠĮłÖÖĘųĢå£ŗH2”¢O2”¢NH3”¢SO2”¢NO2”¢NO”£æÉŅŌĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹÕ¼Æ”£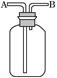
¢ŁČōĘųĢå“ÓBæŚ½ųČė£¬æÉŹÕ¼ÆµÄĘųĢåŹĒ_______________£»
¢ŚČōŌŚÉÕĘæ֊עĀśĖ®£¬ŌņĘųĢåÓ¦øĆ“Ó______£ØĢīŠ“”°A”±»ņ”°B”±£©æŚ½ųČė£¬æÉŅŌŹÕ¼ÆµÄĘųĢåŹĒ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
“ÖŃõ»ÆŠæÖŠŗ¬ÉŁĮæCuO”¢Fe3O4”¢SiO2µČŌÓÖŹ”£¹¤ŅµÉĻŅŌ“ÖŃõ»ÆŠæÉś²śĮņĖįŠæ¾§Ģå£ØZnSO4”¤7H2O£©µÄ¹¤ŅÕĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ ””
””
ŅŃÖŖ£ŗ³£ĪĀĻĀ£¬ČÜŅŗÖŠµÄFe3+”¢Zn2+”¢Fe2+ŅŌĒāŃõ»ÆĪļŠĪŹ½ĶźČ«³ĮµķµÄpH·Ö±šĪŖ£ŗ3.7£¬6.5£¬9.7”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²Ł×÷¢ŪµÄĆū³ĘŹĒ £»
£Ø2£©¼ÓČėŹŹĮæŠæ·ŪµÄ×÷ÓĆĪŖ £»
£Ø3£©¼ÓČė30%H2O2·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ £»
£Ø4£©¼ÓČėŹŹĮæCa(OH)2µ÷½ŚČÜŅŗpH£¬“Ł½ųFe3+Ė®½ā£¬Fe3+Ė®½ā·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½K£½”” ””£¬Ca(OH)2²»ÄܹżĮæµÄŌŅņŹĒ”” ”””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(19·Ö)ŅŌĮņĢśæó(Ö÷ŅŖ³É·ÖĪŖFeS2)ĪŖŌĮĻÖʱøĀČ»ÆĢś¾§Ģå(FeCl3”¤6H2O)µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ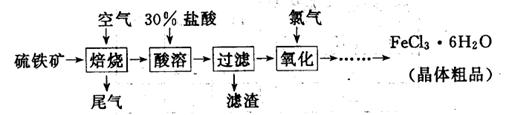
(1)Ńõ»Æ¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ £¬¼ģŃéŃõ»ÆÉś³ÉµÄŃōĄė×ӵďŌ¼ĮŹĒ ”£
(2)Ī²ĘųÖŠÖ÷ŅŖŗ¬N2”¢O2”¢SO2ŗĶÉŁĮæµÄCO2”¢H2O£¬Č”±ź×¼×“æöĻĀµÄĪ²ĘųV L²ā¶ØSO2ŗ¬Įæ£ŗ
·½°øŅ»£ŗČĆĪ²Ęų»ŗĀżĶعżŅŌĻĀ×°ÖĆ”£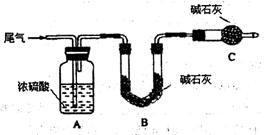
¢ŁCŅĒĘ÷µÄĆū³ĘŹĒ £¬øĆ×°ÖƵÄ×÷ÓĆŹĒ ”£
¢ŚŹµŃ鏱ĻČĶØČėĪ²Ęų£¬ŌŁĶØČėŅ»¶ØĮæµŖĘų”£ČōĶعżB×°ÖƵÄŌöÖŲĄ“²āĮæSO2µÄĢå»ż·ÖŹż”£ÄćČĻĪŖøĆ·½°øŹĒ·ńŗĻĄķ £¬ĒėĖµĆ÷ĄķÓÉ (Čō·½°øŗĻĄķøĆæÕ²»±ŲĢīŠ“)”£
·½°ø¶ž£ŗ½«Ī²Ęų»ŗĀżĶعż×ćĮæäåĖ®£¬ŌŚĖłµĆµÄČÜŅŗÖŠ¼ÓČė¹żĮæĀČ»Æ±µČÜŅŗŗ󣬹żĀĖ£¬½«³ĮµķĻ“µÓ”¢øÉŌļ£¬³ĘµĆĘäÖŹĮæĪŖm g”£
¢Ł¼ÓČė¹żĮæĀČ»Æ±µČÜŅŗµÄÄæµÄŹĒ ”£
¢Ś½ųŠŠ³ĮµķĻ“µÓµÄ·½·ØŹĒ ”£
¢ŪSO2ŗ¬ĮæµÄ±ķ“ļŹ½ŹĒ £ØÓĆŗ¬m”¢VµÄ“śŹżŹ½±ķŹ¾)”£
(3)“ÓFeCl3ČÜŅŗÖŠµĆµ½FeCl3 6H2O¾§ĢåµÄ²Ł×÷°üĄØ ”¢ĄäČ“½į¾§”¢¹żĀĖ£¬øĆ¹ż³ĢŠč±£³ÖŃĪĖį¹żĮ棬½įŗĻ±ŲŅŖµÄĄė×Ó·½³ĢŹ½ĖµĆ÷ŌŅņ .
6H2O¾§ĢåµÄ²Ł×÷°üĄØ ”¢ĄäČ“½į¾§”¢¹żĀĖ£¬øĆ¹ż³ĢŠč±£³ÖŃĪĖį¹żĮ棬½įŗĻ±ŲŅŖµÄĄė×Ó·½³ĢŹ½ĖµĆ÷ŌŅņ .
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖ»ł“”µÄæĘѧ”£
£Ø1£©ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ______£ØĢīŠņŗÅ£©”£
| A£®·ÖŅŗĀ©¶·”¢µĪ¶Ø¹ÜŗĶČŻĮæĘæŹ¹ÓĆĒ°±ŲŠė¼ģ²éŹĒ·ńĀ©Ė® |
| B£®½«µāĖ®µ¹Čė·ÖŅŗĀ©¶·£¬ŌŁ¼ÓŹŹĮæŅŅ“¼£¬³ä·ÖÕńµ“”¢¾²ÖĆ£¬æÉ“ÓµāĖ®ÖŠŻĶČ”µā |
| C£®½ą¾»µÄĢś¶¤ŌŚŹ³ŃĪĖ®ÖŠ½žÅŻŅ»¶ĪŹ±¼ä£¬Ģś¶¤ÉĻÓŠĘųÅŻ£¬ĖµĆ÷Ģś·¢ÉśĮĖĪöĒāøÆŹ“ |
| D£®ĢśĖæŌŚĀČĘųÖŠ¾ēĮŅČ¼ÉÕ£¬»šŠĒĖÄÉä£¬Éś³ÉŗŚÉ«¹ĢĢå |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com