
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЖМЪЧЖЬжмЦкдЊЫиЃЌдзгАыОЖЃКDЃОCЃОAЃОBЁЃвбжЊAЁЂBЭЌжмЦкЃЌAЁЂCЭЌжїзхЃЌCдзгКЫФкЕФжЪзгЪ§ЕШгкAЁЂBдзгКЫФкЕФжЪзгЪ§жЎКЭЃЌCдзгзюЭтВуЕчзгЪ§ЪЧDдзгзюЭтВуЕчзгЪ§ЕФ3БЖЁЃЧыЛиД№ЯТСаЮЪЬтЃК
(1)AдЊЫидкжмЦкБэжаЕФЮЛжУЃК____ЃЛBЁЂDСНдЊЫиаЮГЩЛЏКЯЮяЕФЛЏбЇЪНЃК_________ЁЃ
(2)дЊЫиBЁЂCЁЂDЕФМђЕЅРызгАыОЖЕФДѓаЁЫГађЃК____________(гУРызгЗћКХЛиД№)ЁЃ
(3)CЁЂDСНдЊЫизюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФМюадЧПШѕЙиЯЕЃКЁЁЁЁЁЁЁЁЃОЁЁЁЁЁЁЁЁ(гУЛЏбЇЪНБэЪО)ЁЃ________
(4)BЁЂCаЮГЩЕФЛЏКЯЮядкDдЊЫизюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФШмвКжаЗДгІЕФЛЏбЇЗНГЬЪНЃК____________ЁЃ
ЁОД№АИЁПЕкЖўжмЦкЂѓAзх Na2OЁЂNa2O2 O2->Na+>Al3+ NaOH>Al(OH)3 Al2O3+2NaOH=2NaAlO2+H2O
ЁОНтЮіЁП
AЁЂBЁЂCЁЂDЖМЪЧЖЬжмЦкдЊЫиЃЌдзгАыОЖDЃОCЃОAЃОBЃЌЧвAЁЂBЭЌжмЦкЃЌAЁЂCЭЌжїзхЃЌЭЦжЊAЁЂBЁЂCЁЂDдкжмЦкБэжаЕФДѓжТЯрЖдЮЛжУЮЊЃК![]() ЃЌCЕФдзгКЫФкЕФжЪзгЪ§ЕШгкAЁЂBдзгКЫФкЕФжЪзгЪ§жЎКЭЃЌCжЪзгЪ§=AЕФжЪзгЪ§+8ЃЌЙЪBЮЊ8КХдЊЫибѕЁЃвђAЁЂCЮЊжїзхдЊЫиЃЌЧвCзюЭтВуЕчзгЪ§ЮЊDЕФ3БЖЃЌдђDзюЭтВуЕчзгЪ§жЛФмЮЊ1ЃЌЙЪDЮЊФЦЃЌCЮЊAlЃЌAЮЊХ№ЃЌОнДЫЗжЮіНтД№ЁЃ
ЃЌCЕФдзгКЫФкЕФжЪзгЪ§ЕШгкAЁЂBдзгКЫФкЕФжЪзгЪ§жЎКЭЃЌCжЪзгЪ§=AЕФжЪзгЪ§+8ЃЌЙЪBЮЊ8КХдЊЫибѕЁЃвђAЁЂCЮЊжїзхдЊЫиЃЌЧвCзюЭтВуЕчзгЪ§ЮЊDЕФ3БЖЃЌдђDзюЭтВуЕчзгЪ§жЛФмЮЊ1ЃЌЙЪDЮЊФЦЃЌCЮЊAlЃЌAЮЊХ№ЃЌОнДЫЗжЮіНтД№ЁЃ
ИљОнЩЯЪіЗжЮіЃЌAЮЊBдЊЫиЃЌBЮЊOЃЌCЮЊAlЃЌDЮЊNaдЊЫиЁЃ
(1)AЮЊBдЊЫиЃЌдзгађЪ§ЮЊ5ЃЌЮЛгкжмЦкБэжаЕкЖўжмЦкЂѓAзхЃЛBЁЂDСНдЊЫиаЮГЩЕФЛЏКЯЮяЮЊNa2OЁЂNa2O2ЃЌЙЪД№АИЮЊЃКЕкЖўжмЦкЂѓAзхЃЛNa2OЁЂNa2O2ЃЛ
(2)ЕчзгВуНсЙЙЯрЭЌЕФРызгЃЌКЫЕчКЩЪ§дНДѓЃЌРызгАыОЖдНаЁЃЌРызгЕФЕчзгВудНЖрЃЌРызгАыОЖдНДѓЃЌдђРызгАыОЖДѓаЁЮЊЃКO2-ЃОNa+ЃОAl3+ЃЌЙЪД№АИЮЊЃКO2-ЃОNa+ЃОAl3+ЃЛ
(3)Н№ЪєадNaЃОAlЃЌдђИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЕФМюадЧПШѕЮЊЃКNaOHЃОAl(OH)3ЃЌЙЪД№АИЮЊЃКNaOHЃОAl(OH)3ЃЛ
(4)BЁЂCаЮГЩЕФЛЏКЯЮяЮЊбѕЛЏТСЃЌDдЊЫизюИпМлбѕЛЏЮяЖдгІЫЎЛЏЮяЮЊNaOHЃЌЖўепЗДгІЕФЛЏбЇЗНГЬЪНЃКAl2O3+2NaOHЈT2NaAlO2+H2OЃЌЙЪД№АИЮЊЃКAl2O3+2NaOHЈT2NaAlO2+H2OЁЃ


 ЧсЧЩЖсЙкжмВтдТПМжБЭЈИпПМЯЕСаД№АИ
ЧсЧЩЖсЙкжмВтдТПМжБЭЈИпПМЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЖЯСб1molH2(g)жаЕФHЁЊHМќашвЊЮќЪе436kJФмСПЃЌЖЯСб1molI2(g)жаЕФIЁЊIМќашвЊЮќЪе151kJФмСПЃЌЩњГЩ1molHI(g)жаЕФHЁЊIМќФмЗХГі299kJФмСПЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.1molH2(g)КЭ1molI2(g)ЕФзмФмСПЮЊ587kJ
B.H2(g)+I2(s)![]() 2HI(g) ІЄH=-11kJЁЄmol-1
2HI(g) ІЄH=-11kJЁЄmol-1
C.HI(g)![]()
![]() H2(g)+
H2(g)+![]() I2(g) ІЄH=+5.5kJmol-1
I2(g) ІЄH=+5.5kJmol-1
D.I2(g)БШH2ЗжзгЮШЖЈ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
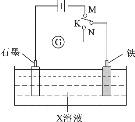
A.KгыNСЌНгЪБЃЌXЮЊСђЫсЃЌвЛЖЮЪБМфКѓШмвКЕФpHМѕаЁ
B.KгыNСЌНгЪБЃЌXЮЊТШЛЏФЦЃЌЪЏФЋЕчМЋЕФЕчЪЦИќИп
C.KгыMСЌНгЪБЃЌXЮЊСђЫсЃЌвЛЖЮЪБМфКѓШмвКЕФpHдіДѓ
D.KгыMСЌНгЪБЃЌXЮЊТШЛЏФЦЃЌЪЏФЋЕчМЋЗДгІЃК4OH--4e-=2H2O+O2Ёќ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЛЏбЇаЫШЄаЁзщгУКЌAЁЂBСНжжН№ЪєЕЅжЪЕФЗлФЉзДЛьКЯЮяНјааШчЯТЪЕбщЃЌЦфзЊЛЏЙиЯЕШчЯТЭМЫљЪОЃЈВПЗжЗДгІЮяКЭЩњГЩЮяЮДСаГіЃЉЃЌЦфжаEЮЊАзЩЋНКзДГСЕэЃЌIЮЊКьКжЩЋГСЕэЁЃЃЈДЫзЊЛЏЙиЯЕжаЫљгУЕФЪдМСЖМЪЧзуСПЕФЃЉ
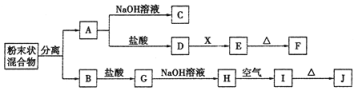
ЃЈ1ЃЉаДГіЯТСаЮяжЪЕФЛЏбЇЪНЃКF____________ЃЌG________________ЁЃ
ЃЈ2ЃЉНЋЛьКЯЮяжаСНжжН№ЪєЗжРыПЊЕФзюМђЕЅЕФЗНЗЈЪЧ___________ЁЃ
ЃЈ3ЃЉDЁњEЕФзЊЛЏжаЃЌМгШыЙ§СПЕФXПЩФмЪЧ_____________________ЁЃ
A.БЅКЭNaClШмвК B.NaOHШмвК C.АБЫЎ D.Ba(OH)2ШмвК
ЃЈ4ЃЉаДГіЯТСазЊЛЏЕФЛЏбЇЗНГЬЪНЃК
AЁњCЃК______________________________________________ЃЛ
HЁњIЃК_______________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЪЕбщаЁзщвРОнЗДгІ![]() ЩшМЦШчЭМ1дЕчГиЃЌЬНОПpHЖдAsO4 3-бѕЛЏадЕФгАЯьЁЃВтЕУЕчбЙгыpHЕФЙиЯЕШчЭМ2ЁЃЯТСагаЙиа№ЪіВЛе§ШЗЕФЪЧ( )
ЩшМЦШчЭМ1дЕчГиЃЌЬНОПpHЖдAsO4 3-бѕЛЏадЕФгАЯьЁЃВтЕУЕчбЙгыpHЕФЙиЯЕШчЭМ2ЁЃЯТСагаЙиа№ЪіВЛе§ШЗЕФЪЧ( )

AЃЎЕїНкpHПЩвдИФБфЗДгІЕФЗНЯђ |
BЃЎpH=0.68ЪБ,ЗДгІДІгкЦНКтзДЬЌ |
CЃЎpH=5ЪБ, ИКМЋЕчМЋЗДгІЪНЮЊ2I-Ѓ2e -= I2 |
DЃЎpHЃО0.68ЪБ,бѕЛЏадI2>AsO43- |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМКЖўЫсЪЧКЯГЩФсСњЃ66ЕФжївЊдСЯжЎвЛЁЃЪЕбщЪвКЯГЩМКЖўЫсЕФдРэЁЂгаЙиЪ§ОнШчЯТЃК
3![]() ЃЋ8HNO3Ёњ3
ЃЋ8HNO3Ёњ3![]() ЃЋ8NOЁќЃЋ7H2O
ЃЋ8NOЁќЃЋ7H2O
ЮяжЪ | ЯрЖдЗжзгжЪСП | УмЖШЃЈ20ЁцЃЉ | ШлЕу | ЗаЕу | ШмНтад |
ЛЗМКДМ | 100 | 0.962 g/cm3 | 25.9Ёц | 160.8Ёц | 20ЁцЪБЃЌдкЫЎжаШмНтЖШЮЊ3.6gЃЌПЩЛьШмгкввДМЁЂБН |
МКЖўЫс | 146 | 1.360 g/cm3 | 152Ёц | 337.5Ёц | дкЫЎжаЕФШмНтЖШЃК15ЁцЪБ1.44gЃЌ25ЁцЪБ2.3gЁЃвзШмгкввДМЃЌВЛШмгкБН |
ВНжшЂёЃКдкШчЭМзАжУЕФШ§ОБЩеЦПжаМгШы16 mL 50%ЕФЯѕЫсЃЈЙ§СПЃЌУмЖШЮЊ1.310 g/cm3ЃЉЃЌдйМгШы1ЁЋ2СЃЗаЪЏЃЌЕЮвКТЉЖЗжаЪЂЗХга5.4 mLЛЗМКДМЁЃ

ВНжшЂђЃКЫЎдЁМгШШШ§ОБЩеЦПжС50ЁцзѓгвЃЌвЦШЅЫЎдЁЃЌЛКТ§ЕЮМг5ЁЋ6ЕЮЛЗМКДМЃЌвЁЖЏШ§ПкЩеЦПЃЌЙлВьЕНгаКьзиЩЋЦјЬхЗХГіЪБдйТ§Т§ЕЮМгЪЃЯТЕФЛЗМКДМЃЌЮЌГжЗДгІЮТЖШдк60ЁцЁЋ65ЁцжЎМфЁЃ
ВНжшЂѓЃКЕБЛЗМКДМШЋВПМгШыКѓЃЌНЋЛьКЯЮягУ80ЁцЁЋ90ЁцЫЎдЁМгШШдМ10 minЃЈзЂвтПижЦЮТЖШЃЉЃЌжБжСЮоКьзиЩЋЦјЬхЩњГЩЮЊжЙЁЃ
ВНжшЂєЃКГУШШНЋЗДгІвКЕЙШыЩеБжаЃЌЗХШыБљЫЎдЁжаРфШДЃЌЮіГіОЇЬхКѓГщТЫЁЂЯДЕгЁЂИЩдяЁЂГЦжиЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉзАжУbЕФУћГЦЮЊ__________ЃЌЪЙгУЪБвЊДг_________ЃЈЬюЁАЩЯПкЁБЛђЁАЯТПкЁБЃЉЭЈШыРфЫЎЃЛЕЮвКТЉЖЗЕФЯИжЇЙмaЕФзїгУЪЧ________________ЁЃ
ЃЈ2ЃЉЪЕбщжаЃЌЯШНЋЮТЖШгЩЪвЮТЩ§жС50ЁцзѓгвЃЌдйТ§Т§ПижЦдк60ЁцЁЋ65ЁцжЎМфЃЌзюКѓПижЦдк80ЁцЁЋ90ЁцЃЌФПЕФЪЧ____________________ЁЃ
ЃЈ3ЃЉБОЪЕбщЫљгУЕФ50%ЕФЯѕЫсЮяжЪЕФСПХЈЖШЮЊ____________ЃЛЪЕбщжаЃЌЕЊбѕЛЏЮяЗЯЦјЃЈжївЊГЩЗжЮЊNOКЭNO2ЃЉПЩвдгУNaOHШмвКРДЮќЪеЃЌЦфжївЊЗДгІЮЊNO+NO2+2NaOH == 2NaNO2+H2OЁЃЦфжаNaOHШмвКПЩвдгУNa2CO3ШмвКРДЬцДњЃЌЧыФЃЗТЩЯЪіЗДгІЃЌаДГіNa2CO3ШмвКЮќЪеЕФЗНГЬЪНЃК______________________________________ЁЃ
ЃЈ4ЃЉЮЊСЫГ§ШЅПЩФмЕФдгжЪКЭМѕЩйВњЦЗЫ№ЪЇЃЌПЩЗжБ№гУБљЫЎЛђ______ЯДЕгОЇЬхЁЃ
ЃЈ5ЃЉЭЈЙ§ГЦСПЕУЕНВњЮя7.00 gЃЌдђБОЪЕбщВњТЪЮЊ__________(ОЋШЗЕН0.1ЃЅ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПClO2КЭNaClO2ОљОпгаЦЏАзадЃЌЙЄвЕЩЯгУClO2ЦјЬхжЦБИNaClO2ЕФЙЄвеСїГЬШчЯТЭМЫљЪОЃК
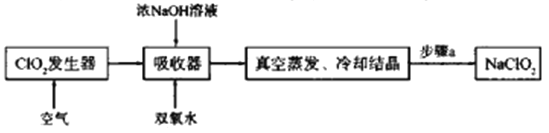
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A. ЙЄвЕЩЯПЩНЋClO2жЦГЩNaClO2ЙЬЬхЃЌБугкжќДцКЭдЫЪф
B. ЭЈШыПеЦјЕФФПЕФЪЧЧ§ИЯГіClO2ЃЌЪЙЦфБЛЮќЪеЦїГфЗжЮќЪе
C. ЮќЪеЦїжаЩњГЩ NaClO2ЕФРызгЗНГЬЪНЃК2ClO2+H2O2=2ClO2Ѓ+O2Ёќ+2H+
D. ВНжшaЕФВйзїАќРЈЙ§ТЫЁЂЯДЕгКЭИЩдя
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙЄвЕЩЯвдШэУЬПѓ(жївЊГЩЗжЪЧMnO2ЃЌКЌгаSiO2ЁЂFe2O3ЕШЩйСПдгжЪ)ЮЊжївЊдСЯжЦБИИпадФмЕФДХадВФСЯЬМЫсУЬ(MnCO3)ЁЃЦфЙЄвЕСїГЬШчЯТЃК

(1)Й§ТЫЂёЫљЕУТЫдќЂёЕФжївЊГЩЗжЮЊ_________(ЬюЛЏбЇЪН)ЁЃ
(2)ЁАбѕЛЏЁБЙ§ГЬжаГ§СЫЗЂЩњMnO2гыSO2ЕФЗДгІЭтЃЌЛЙЗЂЩњСэвЛбѕЛЏЛЙдЗДгІЃЌаДГіИУЗДгІЕФРызгЗНГЬЪНЃК__________________________ЁЃ
(3)ЁАНўУЬЁБЗДгІжаЭљЭљгаИБВњЮяMnS2O6ЩњГЩЃЌЮТЖШЖдЁАНўУЬЁБЗДгІЕФгАЯьШчгвЭМЫљЪОЃЌЮЊМѕЩй MnS2O6 ЕФЩњГЩЃЌЁАНўУЬЁБЕФЪЪвЫЮТЖШЪЧ_______ЁЃ

(4)ЯђЙ§ТЫЂђЫљЕУЕФТЫвКжаМгШыNH4HCO3 ШмвКЪБЮТЖШПижЦдк30-35ЁцЃЌЮТЖШВЛвЫЬЋИпЕФдвђЪЧ_______ЁЃ
(5)МгШыNH4HCO3ШмвККѓЃЌЩњГЩMnCO3ГСЕэЃЌЭЌЪБЛЙгаCO2ЦјЬхЩњГЩЃЌаДГіЗДгІЕФРызгЗНГЬЪНЃК_____________ЁЃ
(6)ЩњГЩЕФMnCO3ГСЕэашОГфЗжЯДЕгЃЌМьбщЯДЕгЪЧЗёЭъШЋЕФЗНЗЈЪЧ______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвдЗЯЬњаМ(КЌгаЩйСПФј)жЦБИИпЬњЫсМи(K2FeO4)ЕФСїГЬШчЯТЭМЫљЪОЃК
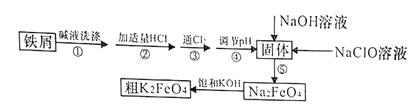
вбжЊЃК25ЁцЪБЃЌвЛаЉН№ЪєЧтбѕЛЏЮяПЊЪМГСЕэКЭЭъШЋГСЕэЪБЕФpHШчЯТБэЫљЪОЃК
M(OH)m | PH | |
ПЊЪМГСЕэ | ГСЕэЭъШЋ | |
Fe (OH)3 | 2.53 | 2.94 |
Ni(OH)2 | 7.60 | 9.75 |
(1)K2FeO4жаЬњдЊЫиЕФЛЏКЯМлЮЊ________________ЁЃ
(2)ЁАМювКЯДЕгЁБЕФФПЕФЪЧГ§ШЅЬњаМБэУцЕФгЭЮлЃЌЪЕМЪвЛАубЁгУNa2CO3ШмвКГ§ЮлЃЌбЁгУNa2CO3ШмвКГ§ЮлЕФдРэЪЧ____________________________(гУРызгЗНГЬЪНБэЪО)ЁЃ
(3)ВНжшЂлЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ___________________ЁЃ
(4)ВНжшЂнЪЧНЋFe(OH)3ЙЬЬхбѕЛЏЮЊNa2FeO4ЃЌЭЌЪБNaClOзЊЛЏЮЊNaClЁЃдђЩњГЩ1mol Na2FeO4ЯћКФNaClOЕФжЪСПЮЊ______gЃЛВНжшЂмЕїНкpHЕФЗЖЮЇЪЧ_______ЁЃ
(5)гУЕЮЖЈЗЈВтЖЈЫљжЦДжK2FeO4ЕФДПЖШ(дгжЪгыKIВЛЗДгІ)ЃКШЁ0.220gДжK2FeO4бљЦЗЃЌМгШызуСПСђЫсЫсЛЏЕФKIШмвКЃЌГфЗжЗДгІКѓЃЌгУ0.200molЁЄLЃ1Na2S2O3БъзМШмвКЕЮЖЈЩњГЩЕФI2ЃЌЕЮЖЈЯћКФБъзМШмвКЕФЬхЛ§ЮЊ20.00mLЁЃЩцМАЕФЗДгІгаЃКFeO42ЃЃЋ4IЃЃЋ8HЃЋЃНFe2ЃЋЃЋ2I2ЃЋ4H2OЃЌ2S2O32ЃЃЋI2ЃНS4O62ЃЃЋ2IЃЁЃ
ЂйЕЮЖЈЪБбЁгУЕФжИЪОМСЮЊ______ЃЌЕЮЖЈжеЕуЕФЯжЯѓЮЊ_____________ЁЃ
ЂкДжK2FeO4ЕФДПЖШЮЊ_____________ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com