
ԭ��صĵ缫���Ʋ�����缫���ϵ������йأ�Ҳ��������Һ�йأ�����˵���в���ȷ����(����)
A����Al��Cu��ϡH2SO4���ԭ��أ��ŵ�ʱSO42����Al�缫�ƶ�
B����Mg��Al��NaOH��Һ���ԭ��أ��为����ӦʽΪ��Al��3e����4OH��===AlO2����2H2O
C����Fe��Cu��FeCl3��Һ���ԭ��أ��为����ӦʽΪ��Cu��2e��===Cu2��
D����Al��Cu��Ũ�������ԭ�������Դ����ʯī�缫�������������Һ��������1 mol Agʱ������ͭ�缫32 g

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б����У��������ǣ� ��
A����ˮ���ȣ�Kw���䣬pH����
B����FeCl3��ˮ��Һ�������ɿɵõ�FeCl3����
C����25 mL��ʽ�ζ�����ȡ20.00mL���������Һ
D���ö��Ե缫���������AgNO3��Һʱ������0.2 mole-ת��ʱ������21.6g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾΪʵ��������ɲ�ͬ�Ļ�ѧʵ����ѡ�õ�װ�û���еIJ���������û�����Դ������
| A �ⶨ�к��� | B ��ʯ��ʯ��ϡ������ȡCO2 | C ����ʯ�� | D ������Һ��ת����Һ |
|
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1mol��L��CH3COOH��Һ�д������µ���ƽ�⣺CH3COOH CH3COO��+H+ ���ڸ�ƽ�⣬����������ȷ���� �� ��
CH3COO��+H+ ���ڸ�ƽ�⣬����������ȷ���� �� ��
A����������NaOH���壬ƽ�����淴Ӧ�����ƶ�
B�������¶ȣ�ƽ��������Ӧ�����ƶ�
C������ˮϡ�ͣ���Һ��c(H+)һ������
D����������CH3COONa���壬ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾΪʵ��������ɲ�ͬ�Ļ�ѧʵ����ѡ�õ�װ�û���еIJ���������û�����Դ������
| A �ⶨ�к��� | B ��ʯ��ʯ��ϡ������ȡCO2 | C ����ʯ�� | D ������Һ��ת����Һ |
|
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״���
( CO2 + 3H2 = CH3OH + H2O )����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء�
��֪ H2(g)�� CO(g) �� CH3OH(l) ��ȼ���ȡ�H�ֱ�Ϊ-285.8 kJ·mol-1��-283.0 kJ·mol-1��-726.5kJ·mol-1����ش��������⣺
(1)��̫���ֽܷ�10molˮ���ĵ�������_____________kJ
(2)���ݻ�Ϊ2L���ܱ������У���CO2��H2�ϳɼ״����������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����________������ţ�
 ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���
���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���
ƽ������Ϊv(CH3OH)= 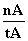 mol·L-1·min-1
mol·L-1·min-1
�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ ����
����
(3)��T1�¶�ʱ����1molCO2��3molH2����һ�ܱպ��������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa,�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ ( �� a ��ʾ )
(4)��֪�״�ȼ�յĻ�ѧ����ʽΪ2CH3OH +3O2 =2CO2 +4H2O ����ֱ���Լ״�Ϊȼ�ϵ���У��������ҺΪ���ԣ������ķ�ӦʽΪ �������ķ�Ӧʽ
Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Һ�������ͺ��Ȼ�����Һ����39%���Ҵ���Һ�����Ȼ��ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������� (����)
A����Һ����ȡ������ B����Һ��������ȡ
C����ȡ������Һ D��������ȡ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��29����˻����ƻ�����Dz����Ǹ�ǿ�ȵ���þ�Ͻ�ij����С��������þ�Ͻ�����о����ⶨ����þ�����������������������ᡢ����������Һ��������ֲ�ͬ��ʵ�鷽����
����һ����þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� �ⶨ������������
�ⶨ������������
����������þ�Ͻ� ��Һ
��Һ
 �������ղ����������
�������ղ����������
(1)д������һ�з�����Ӧ�����ӷ���ʽ ��
(2)ʵ��С����ݷ��������������ʵ��װ�ã�����Ȧ(ͼ�е�����̨��ʡ��)��

����Ϊѡ�� ��ѡ�����)װ�ý���ʵ�������������С��
(3)�÷���������ʵ��ʱ�����˳������ղ��������⣬����������� ��
(4)��չ�о���������þ�Ͻ�������������Һ�м������NaOH��Һʱ�����ɳ��������������NaOH��Һ����Ĺ�ϵ���������ϵ��ʾ��

�����жϣ�������ͼ������������ܷ�����Ͻ���þ����������? (ѡ��ܡ����ܡ�)
���Т٢�����ѡһ������
������������Ͻ���þ��������������˵������ ��
����������Ͻ���þ��������������þ����������Ϊ ������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ�ʻ���������������������� ( )
A����Cl2��H2��Ϻ�ȼ�����Ȼ������壬�ٽ��Ȼ�������ͨ��ˮ�л������
B���Ʊ����ᣬ��ˮ��������������������Ũ����
C�������Ƽ����������̼ͨ�뺬�����Ȼ��Ʊ�����Һ�У����˵õ�̼�����ƾ���
D���õ�������Ȼ����ͱ���ʯ�Ļ����ķ����õ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com