



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
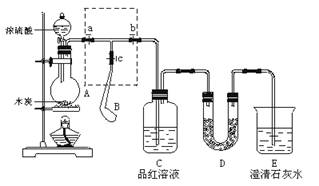
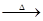 Z+W
Z+W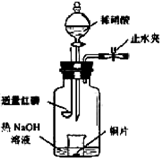
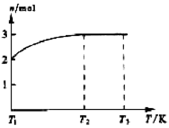
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���ʵ�� | Ԥ��Ŀ�� |
| A | �ѵ�����������ͬ�ִ���ʯ���е�һ���гɷ�ĩ����ͬ�¶��·ֱ�������ͬŨ�ȵ����ᷴӦ���۲�ų���������� | ��֤������Ի�ѧ��Ӧ���ʵ�Ӱ�졣 |
| B | ��װ����ɫ��ͬ��NO2��N2O4��������С�Թܣ��ܷ⣩�ֱ������ˮ����ˮ�У��۲��Թ���������ɫ�仯�� | ��֤�¶ȶԻ�ѧƽ���Ӱ�졣 |
| C | ֱ�ӽ��������ͬ��������þ��Ͷ��ͬ�¶ȵ��з�̪����ˮ�У��۲�������ݵ����ʼ���Һ����ɫ�仯�� | �Ƚ�ͬ���ڽ���Ԫ�صĽ�����ǿ���� |
| D | ��װ��ͬ�ֻ��ǵı�����Һ�������Թ��У��ֱ����Na2CO3��NaHCO3���壬���ȣ��۲���Һ�Ƿ����塣 | �Ƚ�̼�ᡢ���Ӻ�̼���������Ե�ǿ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
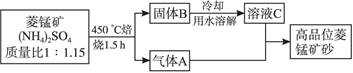
 2KMnO4+2KOH+H2��
2KMnO4+2KOH+H2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 +6HCHO=3H��+6H2O+(CH2)6N4H�� [�ζ�ʱ��1 mol(CH2)6N4H+�� l mol H+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬij��ȤС���ü�ȩ������������ʵ�飺
+6HCHO=3H��+6H2O+(CH2)6N4H�� [�ζ�ʱ��1 mol(CH2)6N4H+�� l mol H+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬij��ȤС���ü�ȩ������������ʵ�飺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
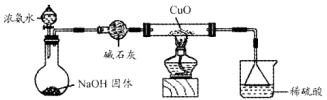
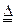 N2+3Cu+3H2O��
N2+3Cu+3H2O��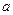 mol������ձ��е���Һ��ʹ��̪��졣�����ݡ���ѧʵ����ƻ���Ҫ������Ʊ������������Һ�ķ����������ʵ�鷽����
mol������ձ��е���Һ��ʹ��̪��졣�����ݡ���ѧʵ����ƻ���Ҫ������Ʊ������������Һ�ķ����������ʵ�鷽���� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com