
Ԫ��X��Y��Z��Wλ��Ԫ�����ڱ���ǰ�����ڣ�ԭ���������������������Ϣ���£�
|
Ԫ�� |
�� �� �� Ϣ |
|
X |
��̬ԭ�Ӻ����������ܼ���ÿ���ܼ��ĵ���������� |
|
Y |
Y��Z����ͬһ���ڣ���ԭ�Ӻ���δ�ɶԵ������ȵ��Ӳ�����1 |
|
Z |
����W�γ����ֻ������ˮ��Һ�������� |
|
W |
�ǵؿ��������ٷֺ����ڶ��Ľ���Ԫ�� |
��ش�
��1��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��__________��Y���ʷ����к� ���м�,�ԱȽ�Y��ͬ�������ڵ�����Ԫ�صĵ�һ�����ܴ�С��ϵ_____>_____>_____(��Ԫ�ط���)
��2��X��Z�γɵ���Ļ�������һ���������ܼ��������� ������ԡ� �����Ǽ��ԡ��� �����ɵ� ������ԡ������Ǽ��ԡ�������
��3��һ��������Z��Y���γ�YZ3��YZ3��ˮ��Ӧ������һ�������һ�������д���÷�Ӧ�ķ���ʽ______________________________
��4��X���γɵ�һ�־�����и�Ӳ�ȡ����۵����ԣ�����______������ӡ��� ��ԭ�ӡ������ӡ������壬�þ���ṹ��Z��Z��Z����Ϊ_______��
 ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
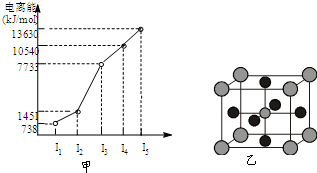 ��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn��
��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�����������Ԫ��X��Y��Z��W��ԭ������������������X��Y��Zλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�W2+��Neԭ�Ӿ�����ͬ�ĵ��Ӳ�ṹ��
��1����X��Y��ɵ�����������ijһȼ�ϵ�ص� ����Ӧ�
��2��Z ������ɵ�ij�ֻ��������ΪDZˮԱ�Ĺ��������û������к��еĻ�ѧ����
��3�����ȷ����У���W�ĵ��������ۡ�����ʳ����ɻ����A��ʹ��ʱ��ˮ����A�У������Ӻ˱����ˣ�������ת���Ƕȿ����ù����ǻ�ѧ��ת��Ϊ �ܣ�д��W��ˮ��Ӧ�Ļ�ѧ����ʽ��
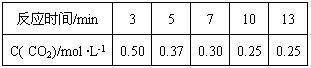
��4��500��ʱ���ܱ������г���1mol��L-1 CO2��3mol ��L-1 H2������Ӧ��
CO2(g)+3H2(g) CH3OH(g)+H2O(g)
����й��������£�
500��ʱ�÷�Ӧ��ƽ�ⳣ��K= ������һλС������ƽ��ʱCO2��ת����Ϊ ���¶����ߣ�Kֵ����������ӦΪ �ȷ�Ӧ��������š�����
��5����֪��298Kʱ��Ca(s) =Ca2��(g) +2e�� ; ��H=+ 1807kJ��mol��1
1/2O2(g)+2e-=O2- (g); ��H=+986kJ.mol-l
Ca2+(g)+O2-( g)= CaO(s) ; ��H=- 3528. 5kJ.mol-l
298Kʱ�������ƺ�������Ӧ����CaO������Ȼ�ѧ����ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������������ѧ����ģ�⿼�Ի�ѧ�� ���ͣ������
��12�֣�����������Ԫ��X��Y��Z��W��ԭ������������������X��Y��Zλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�W2+��Neԭ�Ӿ�����ͬ�ĵ��Ӳ�ṹ��
��1����X��Y��ɵ�����������ijһȼ�ϵ�ص� ����Ӧ�
��2��Z ������ɵ�ij�ֻ��������ΪDZˮԱ�Ĺ��������û������к��еĻ�ѧ����
��3�����ȷ����У���W�ĵ��������ۡ�����ʳ����ɻ����A��ʹ��ʱ��ˮ����A�У������Ӻ˱����ˣ�������ת���Ƕȿ����ù����ǻ�ѧ��ת��Ϊ �ܣ�д��W��ˮ��Ӧ�Ļ�ѧ����ʽ��
��4��500��ʱ���ܱ������г���1mol ��L-1 CO2��3mol ��L-1 H2������Ӧ��
CO2(g)+3H2(g)  CH3OH(g) +H2O(g)
CH3OH(g) +H2O(g)
����й��������£�
500��ʱ�÷�Ӧ��ƽ�ⳣ��K=" " ������һλС������ƽ��ʱCO2��ת����Ϊ ���¶����ߣ�Kֵ����������ӦΪ �ȷ�Ӧ��������š�����
��5����֪��298Kʱ��Ca(s) =Ca2��(g) +2e�� ; ��H="+" 1807kJ��mol��1
1/2O2(g)+2e-= O2- (g); ��H=+986kJ.mol-l
Ca2+(g) +O2-( g)=" CaO(s)" ; ��H="-" 3528. 5kJ.mol-l
298Kʱ�������ƺ�������Ӧ����CaO������Ȼ�ѧ����ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������������ѧ����ģ�⿼�Ի�ѧ�� ���ͣ������
��12�֣�����������Ԫ��X��Y��Z��W��ԭ������������������X��Y��Zλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�W2+��Neԭ�Ӿ�����ͬ�ĵ��Ӳ�ṹ��
��1����X��Y��ɵ�����������ijһȼ�ϵ�ص� ����Ӧ�
��2��Z ������ɵ�ij�ֻ��������ΪDZˮԱ�Ĺ��������û������к��еĻ�ѧ����
��3�����ȷ����У���W�ĵ��������ۡ�����ʳ����ɻ����A��ʹ��ʱ��ˮ����A�У������Ӻ˱����ˣ�������ת���Ƕȿ����ù����ǻ�ѧ��ת��Ϊ �ܣ�д��W��ˮ��Ӧ�Ļ�ѧ����ʽ��
��4��500��ʱ���ܱ������г���1mol ��L-1 CO2��3mol ��L-1 H2������Ӧ��
CO2(g)+3H2(g)
 CH3OH(g)
+H2O(g)
CH3OH(g)
+H2O(g)
����й��������£�

500��ʱ�÷�Ӧ��ƽ�ⳣ��K= ������һλС������ƽ��ʱCO2��ת����Ϊ ���¶����ߣ�Kֵ����������ӦΪ �ȷ�Ӧ��������š�����
��5����֪��298Kʱ��Ca(s) =Ca2��(g) +2e�� ; ��H=+ 1807kJ��mol��1
1/2O2(g)+2e-= O2- (g); ��H=+986kJ.mol-l
Ca2+(g) +O2-( g)= CaO(s) ; ��H=- 3528. 5kJ.mol-l
298Kʱ�������ƺ�������Ӧ����CaO������Ȼ�ѧ����ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��X��Y��Z��W��ԭ������������������X��Y��Zλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�W2+��Neԭ�Ӿ�����ͬ�ĵ��Ӳ�ṹ��
��1����X��Y��ɵ�����������ijһȼ�ϵ�ص� ����Ӧ�
��2��Z ������ɵ�ij�ֻ��������ΪDZˮԱ�Ĺ��������û������к��еĻ�ѧ����
��3�����ȷ����У���W�ĵ��������ۡ�����ʳ����ɻ����A��ʹ��ʱ��ˮ����A�У������Ӻ˱����ˣ�������ת���Ƕȿ����ù����ǻ�ѧ��ת��Ϊ �ܣ�д��W��ˮ��Ӧ�Ļ�ѧ����ʽ��
��4��500��ʱ���ܱ������г���1mol ��L-1 CO2��3mol ��L-1 H2������Ӧ��
CO2(g)+3H2(g) ![]() CH3OH(g) +H2O(g)
CH3OH(g) +H2O(g)
����й��������£�
500��ʱ�÷�Ӧ��ƽ�ⳣ��K= ������һλС������ƽ��ʱCO2��ת����Ϊ ���¶����ߣ�Kֵ����������ӦΪ �ȷ�Ӧ��������š�����
��5����֪��298Kʱ��Ca(s) =Ca2��(g) +2e�� ; ��H=+ 1807kJ��mol��1
1/2O2(g)+2e-= O2- (g); ��H=+986kJ.mol-l
Ca2+(g) +O2-( g)= CaO(s) ; ��H=- 3528. 5kJ.mol-l
298Kʱ�������ƺ�������Ӧ����CaO������Ȼ�ѧ����ʽΪ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com