
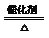 2SO3
2SO3 ”Į100%
”Į100%

 ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
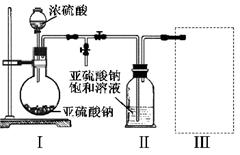

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| Ń”Ļī | ŹµŃéĻÖĻó | ĶĘĀŪ |
| A | ŅŅĻ©ĘųĢåæÉŅŌŹ¹äåĖ®ĶŹÉ« | ŅŅĻ©·¢ÉśČ”“ś·“Ó¦ |
| B | ÅØĮņĖįµēµ¼ĀŹ±Č½ĻµĶ | ĮņĖįÅØ¶Č“óŹ±ŹĒČõµē½āÖŹ |
| C | °±ĘųÓĆÓŚÅēČŖŹµŃé | °±Ęų¼«Ņ×ČÜÓŚĖ® |
| D | ĻõĖįČÜŅŗŹ¹pHŹŌÖ½Ļȱäŗģŗó±ä°× | ĻõĖįĖįŠŌĢ«Ēæ²»ÄÜÓĆpHŹŌÖ½¼ģ²ā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®µćČ¼Įņ»Ē£®²śÉś“Ģ¼¤ŠŌĘųĪ¶ĘųĢ壬øĆĘųĢåæÉŅŌĘÆ°×Ö½½¬ |
| B£®ĻņÕįĢĒÖŠ¼ÓČėÅØĮņĖį£¬ÕįĢĒ±äŗŚÉ«£¬ĖµĆ÷ÅØĮņĖį¾ßÓŠĒæµÄĪüĖ®ŠŌ |
| C£®½«H2SĘųĢåĶØČėCuSO4ČÜŅŗÉś³ÉCuS³Įµķ£¬ĖµĆ÷H2SĪŖĒæµē½āÖŹ |
| D£®½«Alʬ¼ÓČėÅØĮņĖįÖŠ£¬·“Ó¦¾ēĮŅ£¬ĖµĆ÷ÅØĮņĖį¾ßÓŠĒæµÄŃõ»ÆŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ä¾ĢæÓėÅØĻõĖį | B£®ĶÓėĻ”ĻõĖį | C£®ŠæÓėĻ”ĮņĖį | D£®Ä¾ĢæÓėÅØĮņĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 Fe2O3£«SO2”ü£«SO3”ü£¬Čō½«Éś³ÉµÄĘųĢåĶØČėĀČ»Æ±µČÜŅŗÖŠ£¬µĆµ½µÄ³ĮµķĪļŹĒ( )
Fe2O3£«SO2”ü£«SO3”ü£¬Čō½«Éś³ÉµÄĘųĢåĶØČėĀČ»Æ±µČÜŅŗÖŠ£¬µĆµ½µÄ³ĮµķĪļŹĒ( )| A£®BaSO3ŗĶBaSO4 | B£®BaS | C£®BaSO3 | D£®BaSO4 |
²éæ““š°øŗĶ½āĪö>>
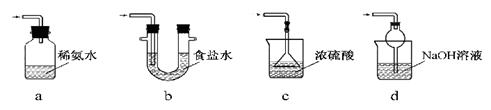
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
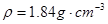 £© 60mL³ä·Ö·“Ó¦£¬ŠæČ«²æČܽā£¬¶ŌÓŚÖʵƵÄĘųĢ壬ӊĶ¬Ń§ČĻĪŖæÉÄÜ»ģÓŠŌÓÖŹ”£
£© 60mL³ä·Ö·“Ó¦£¬ŠæČ«²æČܽā£¬¶ŌÓŚÖʵƵÄĘųĢ壬ӊĶ¬Ń§ČĻĪŖæÉÄÜ»ģÓŠŌÓÖŹ”£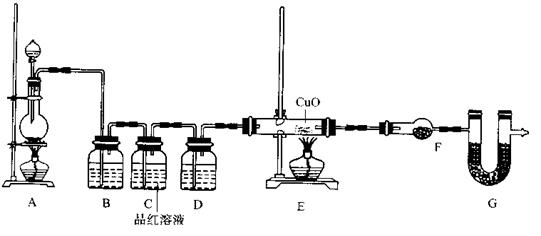
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

| A£®Ė® | B£®±„ŗĶNaHSO3ČÜŅŗ | C£®ĖįŠŌKMnO4ČÜŅŗ | D£®NaOHČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com