
Ņ»¶ØĪĀ¶ČĻĀ£¬ÓŠa”¢ŃĪĖį£»b”¢ĮņĖį£»c”¢“×ĖįČżÖÖĖįµÄĻ”ČÜŅŗ”££ØÓĆa”¢b”¢c”¢<”¢=”¢>ŗÅĢīŠ“£©
¢Łµ±ĘäĪļÖŹµÄĮæÅضČĻąĶ¬Ź±£¬c(H+)Óɓ󵽊”µÄĖ³ŠņŹĒ_______________ ____£¬
¢Śµ±Ęäc(H+)ĻąĶ¬Ź±£¬ĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ________________ _____£¬
¢Ūµ±c(H+)ĻąĶ¬”¢Ģå»żĻąĶ¬Ź±£¬·Ö±š¼ÓČė×ćĮæŠæ£¬ĻąĶ¬×“æö²śÉśµÄĘųĢåĢå»żÓɓ󵽊”µÄĖ³ŠņĪŖ__________ ”£
¢Üµ±pHĻąĶ¬”¢Ģå»żĻąĶ¬Ź±£¬Ķ¬Ź±¼ÓČėŠæ£¬Čō²śÉśĻąĶ¬Ģå»żµÄĒāĘų£ØĻąĶ¬×“æö£©ŌņæŖŹ¼Ź±·“Ó¦ĖŁĀŹ__________________£¬·“Ó¦ĖłŠčµÄŹ±¼ä__________ _____ ____”£
¢Ż½«c(H+)ĻąĶ¬µÄČżÖÖĖį¾łĻ”ŹĶ10±¶£¬pHÓɓ󵽊”µÄĖ³ŠņĪŖ_____________ _______”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ



| 1 | 18 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĻĀĶ¼µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬ŅŃÖŖAŹĒÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĖįŹ½ŃĪ”£D”¢Y”¢HĪŖĘųĢ壬XĪŖĪŽÉ«ŅŗĢ壬GŗĶK¾łŹĒ³£¼ūµÄĒæĖį”£HÓėNa2O2æÉ·¢Éś»ÆŗĻ·“Ó¦![]() £¬Éś³ÉµÄŃĪÓėBa2+·“Ó¦æÉÉś³É²»ČÜÓŚĻ”GµÄ°×É«³Įµķ£¬Ņ»øöD·Ö×ÓÖŠŗ¬ÓŠ10øöµē×Ó”£
£¬Éś³ÉµÄŃĪÓėBa2+·“Ó¦æÉÉś³É²»ČÜÓŚĻ”GµÄ°×É«³Įµķ£¬Ņ»øöD·Ö×ÓÖŠŗ¬ÓŠ10øöµē×Ó”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©DµÄµē×ÓŹ½ĪŖ£ŗ___________________£»
£Ø2£©Š“³öD£«H£«X”śAµÄ»Æѧ·½³ĢŹ½£ŗ________£»
£Ø3£©Š“³öC”śHµÄĄė×Ó·½³ĢŹ½£ŗ_______________£»
£Ø4£©Š“³öDÓėK·“Ӧɜ³ÉµÄÕżŃĪČÜŅŗÖŠµÄĄė×ÓÅØ¶Č“óŠ”¹ŲĻµ£ŗ____________________£»
£Ø5£©ŅŃÖŖ1molH£Øg£©ĶźČ«×Ŗ»ÆĪŖI£Øg£©Ź±·ÅČČ98.3kJ£¬Ōņ“Ė·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ_________£»
ijĢõ¼žĻĀ£¬µ±¼ÓČė4 mol HŗĶ2 mol Yŗ󣬷ųö314.56 kJµÄČČŹ±£¬“ĖŹ±HµÄ×Ŗ»ÆĀŹĪŖ__________£»
£Ø6£©Ņ»¶ØĪĀ¶ČĻĀ£¬ÓŠæÉÄę·“Ó¦£ŗaD£Øg£©£«bY£Øg£©![]() cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol DŗĶ5 mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol DŗĶ5 mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗŚĮś½Ź”“óĒģŹŠČżŹ®ĪåÖŠøßČżĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©ČēĻĀĶ¼µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬ŅŃÖŖAŹĒÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĖįŹ½ŃĪ”£D”¢Y”¢HĪŖĘųĢ壬XĪŖĪŽÉ«ŅŗĢ壬GŗĶK¾łŹĒ³£¼ūµÄĒæĖį”£HÓėNa2O2æÉ·¢Éś»ÆŗĻ·“Ó¦£¬Éś³ÉµÄŃĪÓėBa2+·“Ó¦æÉÉś³É²»ČÜÓŚĻ”GµÄ°×É«³Įµķ£¬Ņ»øöD·Ö×ÓÖŠŗ¬ÓŠ10øöµē×Ó”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©DµÄµē×ÓŹ½ĪŖ£ŗ___________________£»
£Ø2£©Š“³öD£«H£«X”śAµÄ»Æѧ·½³ĢŹ½£ŗ________________________________£»
£Ø3£©Š“³öC”śHµÄĄė×Ó·½³ĢŹ½£ŗ_______________________________£»
£Ø4£©Š“³öDÓėK·“Ӧɜ³ÉµÄÕżŃĪµÄ»ÆѧŹ½£ŗ_____________£»
£Ø5£©ŅŃÖŖ1molH£Øg£©ĶźČ«×Ŗ»ÆĪŖI£Øg£©Ź±·ÅČČ98.3kJ£¬Ōņ“Ė·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ____________________________£»Ä³Ģõ¼žĻĀ£¬µ±¼ÓČė4 mol HŗĶ2 mol Yŗ󣬷ųö314.56 kJµÄČČŹ±£¬“ĖŹ±HµÄ×Ŗ»ÆĀŹĪŖ__________£»
£Ø6£©Ņ»¶ØĪĀ¶ČĻĀ£¬ÓŠæÉÄę·“Ó¦£ŗaD£Øg£©£«bY£Øg£© cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol DŗĶ5 mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol DŗĶ5 mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗŚĮś½Ź”“óĒģŹŠøßČżĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©ČēĻĀĶ¼µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬ŅŃÖŖAŹĒÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĖįŹ½ŃĪ”£D”¢Y”¢HĪŖĘųĢ壬XĪŖĪŽÉ«ŅŗĢ壬GŗĶK¾łŹĒ³£¼ūµÄĒæĖį”£HÓėNa2O2æÉ·¢Éś»ÆŗĻ·“Ó¦£¬Éś³ÉµÄŃĪÓėBa2+·“Ó¦æÉÉś³É²»ČÜÓŚĻ”GµÄ°×É«³Įµķ£¬Ņ»øöD·Ö×ÓÖŠŗ¬ÓŠ10øöµē×Ó”£
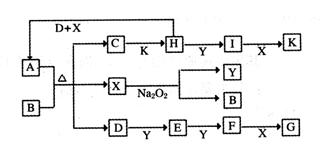
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©DµÄµē×ÓŹ½ĪŖ£ŗ___________________£»
£Ø2£©Š“³öD£«H£«X”śAµÄ»Æѧ·½³ĢŹ½£ŗ________________________________£»
£Ø3£©Š“³öC”śHµÄĄė×Ó·½³ĢŹ½£ŗ_______________________________£»
£Ø4£©Š“³öDÓėK·“Ӧɜ³ÉµÄÕżŃĪµÄ»ÆѧŹ½£ŗ_____________£»
£Ø5£©ŅŃÖŖ1molH£Øg£©ĶźČ«×Ŗ»ÆĪŖI£Øg£©Ź±·ÅČČ98.3kJ£¬Ōņ“Ė·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ____________________________£»Ä³Ģõ¼žĻĀ£¬µ±¼ÓČė4 mol HŗĶ2 mol Yŗ󣬷ųö314.56 kJµÄČČŹ±£¬“ĖŹ±HµÄ×Ŗ»ÆĀŹĪŖ__________£»
£Ø6£©Ņ»¶ØĪĀ¶ČĻĀ£¬ÓŠæÉÄę·“Ó¦£ŗaD£Øg£©£«bY£Øg£© cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol
DŗĶ5 mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol
DŗĶ5 mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗŚĮś½Ź”¹ž¶ū±õŹŠ2010½ģøßČżŅ»Ä££ØĄķæĘ×ŪŗĻ£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©
ČēĻĀĶ¼µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬ŅŃÖŖAŹĒÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĖįŹ½ŃĪ”£D”¢Y”¢HĪŖĘųĢ壬XĪŖĪŽÉ«ŅŗĢ壬GŗĶK¾łŹĒ³£¼ūµÄĒæĖį”£HÓėNa2O2æÉ·¢Éś»ÆŗĻ·“Ó¦£¬Éś³ÉµÄŃĪÓėBa2+·“Ó¦æÉÉś³É²»ČÜÓŚĻ”GµÄ°×É«³Įµķ£¬Ņ»øöD·Ö×ÓÖŠŗ¬ÓŠ10øöµē×Ó”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
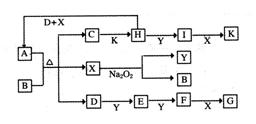
£Ø1£©DµÄµē×ÓŹ½ĪŖ£ŗ___________________£»
£Ø2£©Š“³öD£«H£«X”śAµÄ»Æѧ·½³ĢŹ½£ŗ________£»
£Ø3£©Š“³öC”śHµÄĄė×Ó·½³ĢŹ½£ŗ_______________£»
£Ø4£©Š“³öDÓėK·“Ӧɜ³ÉµÄÕżŃĪČÜŅŗÖŠµÄĄė×ÓÅØ¶Č“óŠ”¹ŲĻµ£ŗ____________________£»
£Ø5£©ŅŃÖŖ1molH£Øg£©ĶźČ«×Ŗ»ÆĪŖI£Øg£©Ź±·ÅČČ98.3kJ£¬Ōņ“Ė·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ_________£»
ijĢõ¼žĻĀ£¬µ±¼ÓČė4 mol HŗĶ2 mol Yŗ󣬷ųö314.56 kJµÄČČŹ±£¬“ĖŹ±HµÄ×Ŗ»ÆĀŹĪŖ__________£»
£Ø6£©Ņ»¶ØĪĀ¶ČĻĀ£¬ÓŠæÉÄę·“Ó¦£ŗaD£Øg£©£«bY£Øg£© cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol DŗĶ5
mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
cE£Øg£©£«dX£Øg£©”£ŌŚ2 LĆܱÕČŻĘ÷ÖŠ£¬³äČė4 mol DŗĶ5
mol Y£¬ÓŠ“߻ƼĮ“ęŌŚĻĀ£¬2 minŗó·“Ó¦“ļµ½Ę½ŗā£¬²āµĆĘ½ŗāŹ±ČŻĘ÷ÄŚµÄŃ¹Ēæ±Č·“Ó¦Ē°Ōö¼ÓĮĖ1£Æ18”£ŌņĒ°2 minÄŚÓĆE±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ______mol”¤L£1”¤min-1£¬Ę½ŗāŹ±DµÄÅضČĪŖ________mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com