
 £»
£» £»
£»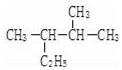 µÄĆū³Ę£ØĻµĶ³ĆüĆū·Ø£©2£¬3-¶ž¼×»łĪģĶ飮
µÄĆū³Ę£ØĻµĶ³ĆüĆū·Ø£©2£¬3-¶ž¼×»łĪģĶ飮 ·ÖĪö £Ø1£©ŅŅĻ©·Ö×ÓÖŠĢ¼Ģ¼ŅŌĖ«¼üĻąĮ¬£¬Ģ¼Ź£Óą¼Ū¼ü±»H±„ŗĶ£¬ÓÉ“ĖŠ“³öµē×ÓŹ½£»
£Ø2£©øł¾ŻCŌŖĖŲ”¢HŌŖĖŲŹŲŗćČ·¶ØøĆĢžµÄ·Ö×ÓŹ½ĪŖC6H12£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀÓėH2·¢Éś¼Ó³É·“Ó¦£¬Éś³É2£¬2-¶ž¼×»ł¶”Ķ飬ŌņøĆĢžµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©3C-CH=CH2£»
£Ø3£©Ģž1molÓė2mol HClĶźČ«¼Ó³É£¬ŌņøĆĢž·Ö×ÓÓŠ2øöĖ«¼ü»ņ1øöČż¼ü£¬1molĀČ“śĶéÄÜŗĶ4molĀČĘų·¢ÉśĶźČ«Č”“ś·“Ó¦£¬ŌņĀČ“śĶé·Ö×ÓÖŠÓŠ4øöHŌ×Ó£¬ĖłŅŌŌĢž·Ö×ÓÖŠÓŠ2øöHŌ×Ó£¬¾Ż“ĖČ·¶Ø£»
£Ø4£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖ72µÄĶéĢž£¬ĶéĢžĶØŹ½ĪŖCnH2n+2£»12n+2n+2=72£¬¼ĘĖćµĆµ½n=5£¬·Ö×ÓŹ½ĪŖ£ŗC5H12£»
ĪģĶéµÄĶ¬·ÖŅģ¹¹ĢåÓŠCH3-CH2-CH2-CH2-CH3£¬ £¬
£¬ £¬¼«ŠŌ·Ö×ÓµÄČŪ·Šµć“óÓŚ·Ē¼«ŠŌ·Ö×Ó£¬¾Ż“Ė½ųŠŠ·ÖĪö£»
£¬¼«ŠŌ·Ö×ÓµÄČŪ·Šµć“óÓŚ·Ē¼«ŠŌ·Ö×Ó£¬¾Ż“Ė½ųŠŠ·ÖĪö£»
£Ø5£©ÅŠ¶ĻÓŠ»śĪļµÄĆüĆūŹĒ·ńÕżČ·»ņ¶ŌÓŠ»śĪļ½ųŠŠĆüĆū£¬ĘäŗĖŠÄŹĒ×¼Č·Ąķ½āĆüĆū¹ę·¶£ŗĶéĢžĆüĆūŌŌņ£ŗ
¢Ł³¤-----Ń”×ī³¤Ģ¼Į“ĪŖÖ÷Į“£»
¢Ś¶ą-----ÓöµČ³¤Ģ¼Į“Ź±£¬Ö§Į“×ī¶ąĪŖÖ÷Į“£»
¢Ū½ü-----ĄėÖ§Į“×ī½üŅ»¶Ė±ąŗÅ£»
¢ÜŠ”-----Ö§Į“±ąŗÅÖ®ŗĶ×īŠ”£®æ“ĻĀĆę½į¹¹¼ņŹ½£¬“ÓÓŅ¶Ė»ņ×ó¶Ėæ“£¬¾ł·ūŗĻ”°½ü-----ĄėÖ§Į“×ī½üŅ»¶Ė±ąŗÅ”±µÄŌŌņ£»
¢Ż¼ņ-----Į½Č”“ś»ł¾ąĄėÖ÷Į“Į½¶ĖµČ¾ąĄėŹ±£¬“Ó¼ņµ„Č”“ś»łæŖŹ¼±ąŗÅ£®ČēČ”“ś»ł²»Ķ¬£¬¾Ķ°Ń¼ņµ„µÄŠ“ŌŚĒ°Ćę£¬ø“ŌӵĊ“ŌŚŗóĆę£®
ÓŠ»śĪļµÄĆū³ĘŹéŠ“ŅŖ¹ę·¶£»¶ŌÓŚ½į¹¹ÖŠŗ¬ÓŠ±½»·µÄ£¬ĆüĆūŹ±æÉŅŌŅĄ“Ī±ąŗÅĆüĆū£¬Ņ²æÉŅŌøł¾ŻĘäĻą¶ŌĪ»ÖĆ£¬ÓĆ”°ĮŚ”±”¢”°¼ä”±”¢”°¶Ō”±½ųŠŠĆüĆū£»ŗ¬ÓŠ¹ŁÄÜĶŵÄÓŠ»śĪļĆüĆūŹ±£¬ŅŖŃ”ŗ¬¹ŁÄÜĶŵÄ×ī³¤Ģ¼Į“×÷ĪŖÖ÷Į“£¬¹ŁÄÜĶŵÄĪ»“Ī×īŠ”£®
½ā“š ½ā£ŗ£Ø1£©ŅŅĻ©·Ö×ÓÖŠĢ¼Ģ¼ŅŌĖ«¼üĻąĮ¬£¬Ģ¼Ź£Óą¼Ū¼ü±»H±„ŗĶ£¬ÓÉ“ĖŠ“³öµē×ÓŹ½ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø2£©n£ØĢž£©£ŗn£ØC£©£ŗn£ØH£©=n£ØĢž£©£ŗn£ØCO2£©£ŗ2n£ØH2O£©=0.1mol£ŗ0.6mol£ŗ0.6mol”Į2=1£ŗ6£ŗ12£¬¼“1øö·Ö×ÓÖŠŗ¬ÓŠ6øöCŌ×Ó”¢12øöHŌ×Ó£¬¹ŹøĆĢžµÄ·Ö×ÓŹ½ĪŖC6H12£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀÓėH2·¢Éś¼Ó³É·“Ó¦£¬Éś³É2.2-¶ž¼×»ł¶”Ķ飬ŌņøĆĢžµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©3C-CH=CH2£¬
¹Ź“š°øĪŖ£ŗ£ØCH3£©3C-CH=CH2£»
£Ø3£©Ģž1molÓė2mol HClĶźČ«¼Ó³É£¬ŌņøĆĢž·Ö×ÓÓŠ2øöĖ«¼ü»ņ1øöČż¼ü£¬1molĀČ“śĶéÄÜŗĶ4molĀČĘų·¢ÉśĶźČ«Č”“ś·“Ó¦£¬ŌņĀČ“śĶé·Ö×ÓÖŠÓŠ4øöHŌ×Ó£¬ĀČ“śĶé·Ö×ÓÖŠÓŠ2øöHŌ×ÓŹĒĢžÓėĀČ»ÆĒā¼Ó³ÉŅżČėµÄ£¬ĖłŅŌŌĢž·Ö×ÓÖŠÓŠ2øöHŌ×Ó£¬¹ŹøĆĢžĪŖCH”ŌCH£¬
¹Ź“š°øĪŖ£ŗCH”ŌCH£»
£Ø4£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖ72µÄĶéĢž£¬ĶéĢžĶØŹ½ĪŖCnH2n+2£»12n+2n+2=72£¬¼ĘĖćµĆµ½n=5£¬·Ö×ÓŹ½ĪŖ£ŗC5H12£»
ĪģĶéµÄĶ¬·ÖŅģ¹¹ĢåÓŠCH3-CH2-CH2-CH2-CH3£¬ £¬
£¬ £¬¼«ŠŌ·Ö×ÓµÄČŪ·Šµć“óÓŚ·Ē¼«ŠŌ·Ö×Ó£¬¹ŹČŪ·Šµć×īŠ”µÄĪŖ
£¬¼«ŠŌ·Ö×ÓµÄČŪ·Šµć“óÓŚ·Ē¼«ŠŌ·Ö×Ó£¬¹ŹČŪ·Šµć×īŠ”µÄĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø5£©ĶéĢžĆüĆū£¬Ń”Ö÷Į“ĪŖ5øö£¬“ÓĄėČ”“ś»ł½üµÄŅ»¶Ė±ąŗÅ£¬Š“Ćū³ĘĪŖ£ŗ2£¬3-¶ž¼×»łĪģĶ飬
¹Ź“š°øĪŖ£ŗ2£¬3-¶ž¼×»łĪģĶ飮
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻ£¬µē×ÓŹ½µÄŹéŠ“£¬ĆüĆū¼°Ķ¬·ÖŅģ¹¹ĢåµÄ·ÖĪöÅŠ¶Ļ£¬ÄѶČÖŠµČ£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
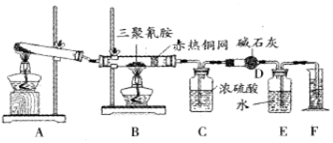
| ŅĒĘ÷ | C | D |
| ŹµŃéĒ° | 101.0g | 56.0g |
| ŹŌŃéŗó | 106.4g | 69.2g |
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 £¬ĘäŗĖ“Ź²ÕńĒāĘ×ÖŠÓŠ2øöŠÅŗţزĪ¼ūĶ¼1£©£®
£¬ĘäŗĖ“Ź²ÕńĒāĘ×ÖŠÓŠ2øöŠÅŗţزĪ¼ūĶ¼1£©£®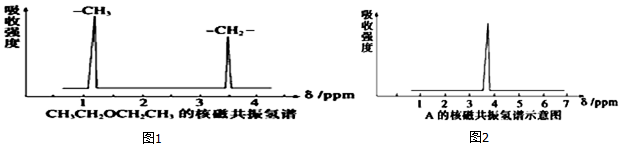
²éæ““š°øŗĶ½āĪö>>
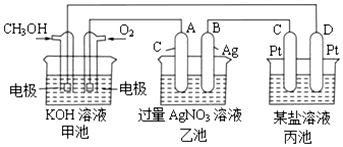
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
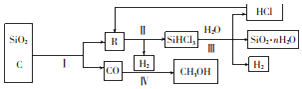
 ŅŃÖŖ¼×“¼”¢ŅŅ“¼¶¼ŹĒÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
ŅŃÖŖ¼×“¼”¢ŅŅ“¼¶¼ŹĒÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ| »Æѧ·“Ó¦¼°Ę½ŗā³£Źż | Ę½ŗā³£ŹżŹżÖµ | ||
| 500”ę | 800”ę | ||
| ¢Ł2H2£Øg£©+CO£Øg£©?CH3OH£Øg£© | K1 | 2.5 | 0.15 |
| ¢ŚH2£Øg£©+CO2£Øg£©?H2O£Øg£©+CO£Øg£© | K2 | 1.0 | 2.50 |
| ¢Ū3H2£Øg£©+CO2£Øg£©?CH3OH£Øg£©+H2O£Øg£© | K3 | 2.5 | 0.375 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µē½āČŪČŚµÄAlCl3Éś²śĀĮµ„ÖŹ | |
| B£® | ½«Cl2ÓėHCl»ģŗĻĘųĢåĶعż±„ŗĶŹ³ŃĪĖ®æɵƵ½“æ¾»µÄCl2 | |
| C£® | ÓĆBa£ØOH£©2ČÜŅŗæɼų±šNaCl”¢AlCl3”¢NH4Cl”¢Na2SO4ĖÄÖÖČÜŅŗ | |
| D£® | ÓĆ·ÖŅŗĀ©¶·“ÓŹ³“×ÖŠ·ÖĄė³öŅŅĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com