


 ����98%��ŨH2SO4�����ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������0.5mol?L-1��ϡH2SO41000mL��ҪŨ����������
����98%��ŨH2SO4�����ʵ���Ũ�ȣ��ٸ���ϡ�Ͷ��ɼ�������0.5mol?L-1��ϡH2SO41000mL��ҪŨ����������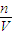 ������������ҺŨ�ȵ�Ӱ�죮
������������ҺŨ�ȵ�Ӱ�죮 mol/L=18.4mol/L������ҪŨ��������ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬��V×18.4mol/L=0.5mol?L-1×1000mL�����V=27.2mL��
mol/L=18.4mol/L������ҪŨ��������ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬��V×18.4mol/L=0.5mol?L-1×1000mL�����V=27.2mL��
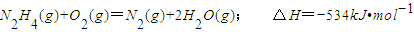


 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��һ��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com