
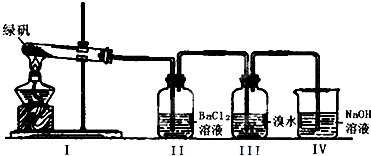
| ʵ�鲽�� | ʵ��Ԥ�������� |
| ����һ��ȡ������FeSO4�������Թ��У�����һ����ˮ�ܽ⣮ | \ |
| ������� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ϊͬ���칹�� |
| B��������Ȼ�л��߷��ӻ����� |
| C�����⣨I2���������ɫ |
| D���������ھ��ᷢ��ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
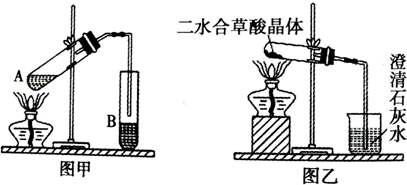
| 175�� |
| �¶�/�� | 25 | 300 | 350 | 400 | 500 | 600 | 900 |
| ��������/g | 1.000 | 0.800 | 0.800 | 0.400 | 0.444 | 0.444 | 0.429 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �� | �� | �� | �� |
| װ�� |  |  | 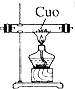 |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
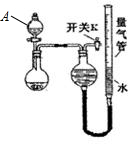
| ʵ�� | ҩƷ | ��ȡ���� | ��������Һ�� |
| �� | Cu��ϡHNO3 | NO | H2O |
| �� | NaOH��s����Ũ��ˮ | NH3 | |
| �� | þ���Ͻ�NaOH��Һ | H2 | H2O |

| ��� | þ���Ͻ���� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| �� | 1.0g | 10.0mL | 346.3mL |
| �� | 1.0g | 10.0mL | 335.0mL |
| �� | 1.0g | 10.0mL | 345.7mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
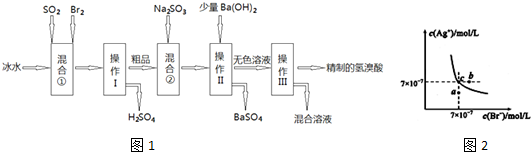
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����γ�һ������ |
| B�����ϵ��µ��ʵ����������� |
| C�����ϵ����⻯��ķе������� |
| D�����ϵ����⻯����ȶ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com