
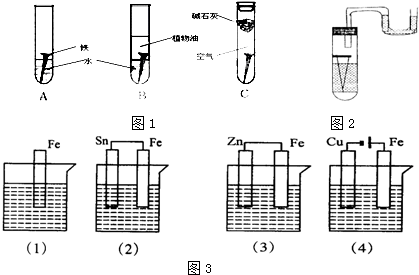
·ÖĪö £Ø1£©¢ŁĢśŌŚ³±ŹŖµÄæÕĘųÖŠŅ×·¢Éśµē»ÆѧøÆŹ“£¬øō¾ųæÕĘų»ņŌŚøÉŌļµÄæÕĘųÖŠÄŃŅŌŠĪ³ÉŌµē³Ų·“Ó¦£»
¢ŚĢś·¢Éśµē»ÆѧøÆŹ“Ź±£¬Õż¼«ÉĻŹĒŃõĘų·¢ÉśµĆµē×ӵĻ¹Ō·“Ó¦£»
¢Ū½«Ė®Öó·ŠæÉŅŌ½«Ė®ÖŠµÄæÕĘųÅųö£¬Ö²ĪļÓĶŗĶĖ®ŹĒ»„²»ĻąČܵģ»
¢Ü¼īŹÆ»ŅŹĒæÉŅŌĪüĖ®µÄ£»
£Ø2£©ÉśĢśÖŠŗ¬ÓŠĢ¼£¬Ģś”¢Ģ¼ŗĶŗĻŹŹµÄµē½āÖŹČÜŅŗ¹¹³ÉŌµē³Ų£¬ŌŚČõĖįŠŌ»ņÖŠŠŌĢõ¼žĻĀ£¬Ģś·¢ÉśĪüŃõøÆŹ“£¬øŗ¼«ÉĻĢśŹ§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬Õż¼«ÉĻŃõĘųµĆµē×ÓŗĶĖ®·“Ӧɜ³ÉĒāŃõøłĄė×Ó£¬ŌŚĖįŠŌĢõ¼žĻĀ£¬Ģś·¢ÉśĪöĒāøÆŹ“£¬øŗ¼«ÉĻĢśŹ§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬Õż¼«ÉĻĒāĄė×ӵƵē×Ó·¢Éś»¹Ō·“Ó¦£»
£Ø3£©½šŹō±»øÆŹ“æģĀżĖ³ŠņĪŖ£ŗ×÷µē½ā³ŲŃō¼«£¾×÷Ōµē³Ųøŗ¼«£¾»ÆѧøÆŹ“£¾×÷Ōµē³ŲÕż¼«£¾×÷µē½ā³ŲŅõ¼«£¬¾Ż“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©¢ŁĢśÉśŠāµÄĶā²æĢõ¼žŹĒ½šŹōŅŖŗĶæÕĘųÖŠµÄĖ®ŅŌ¼°ŃõĘų½Ó“„£¬¹Ź“š°øĪŖ£ŗŗĶæÕĘųŅŌ¼°Ė®½Ó“„£»
¢ŚĢś¶¤·¢Éśµē»ÆøÆŹ“£¬øŗ¼«ÉĻĢśĪŖ»īĘĆ½šŹō£¬Ņ׏§Č„µē×Ó¶ų±»Ńõ»Æ£¬Õż¼«ÉĻŹĒŃõĘų·¢ÉśµĆµē×ӵķ“Ó¦O2+2H2O+4e-=4OH-£¬¹Ź“š°øĪŖ£ŗO2+2H2O+4e-=4OH-£»
¢ŪĖ®ÖŠČܽāÓŠŅ»¶ØµÄæÕĘų£¬Öó·ŠæÉŅŌ½«æÕĘųÅųö£¬Ö²ĪļÓĶŗĶĖ®ŹĒ»„²»ĻąČܵģ¬ĖüµÄ×÷ÓĆŹĒøō¾ųæÕĘųÖŠµÄŃõĘų£¬¹Ź“š°øĪŖ£ŗÖó·Š£»øō¾ųæÕĘųÖŠµÄŃõĘų£»
¢Ü¼īŹÆ»ŅÄÜĪüĖ®£¬ĖüµÄ×÷ÓĆŹĒĪüŹÕæÕĘųÖŠµÄĖ®ÕōĘų£¬¹Ź“š°øĪŖ£ŗĪüŹÕæÕĘųÖŠµÄĖ®ÕōĘų£»
£Ø2£©ÉśĢśÖŠŗ¬ÓŠĢ¼£¬Ģś”¢Ģ¼ŗĶŗĻŹŹµÄµē½āÖŹČÜŅŗ¹¹³ÉŌµē³Ų£¬
¢Ł¹ÜÖŠŅŗÖł×ó±ßĻĀ½µ£¬ÓŅ±ßÉĻÉż£¬ĖµĆ÷½šŹōµÄøÆŹ“ÖŠÉś³ÉĘųĢåµ¼ÖĀŃ¹ĒæŌö“ó£¬Ōņ½šŹō·¢ÉśµÄŹĒĪöĒāøÆŹ“£¬ČÜŅŗ³ŹĖįŠŌ£¬øŗ¼«ÉĻĢśŹ§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬µē¼«·“Ó¦Ź½ĪŖ£ŗFe-2e-ØTFe2+£¬Õż¼«ÉĻĒāĄė×ӵƵē×Ó·¢Éś»¹Ō·“Ó¦£¬µē¼«·“Ó¦Ź½ĪŖ£ŗ4H++4e-ØT2H2”ü£¬¹Ź“š°øĪŖ£ŗĖįŠŌ£»4H++4e-ØT2H2”ü£»
¢Śµ¼¹ÜÖŠŅŗÖłÓŅ±ßĻĀ½µ£¬×ó±ßÉĻÉż£¬ĖµĆ÷µē½āÖŹ»·¾³ŹĒČõĖįŠŌ»ņŹĒÖŠŠŌ£¬ŹŌ¹ÜÄŚĘųĢåŅņŗĶĖ®·“Ó¦¶ųŹ¹ĘäŃ¹Ēæ¼õŠ”£¬·¢ÉśµÄŹĒĪüŃõøÆŹ“£¬Ģś×÷øŗ¼«£¬øŗ¼«ÉĻĢśŹ§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬µē¼«·“Ó¦Ź½ĪŖ£ŗFe-2e-ØTFe2+£¬Õż¼«ÉĻŃõĘųµĆµē×Ó·¢Éś»¹Ō·“Ó¦£¬µē¼«·“Ó¦Ź½ĪŖ2H2O+O2+4e-ØT4OH-£¬
¹Ź“š°øĪŖ£ŗĪüŃõøÆŹ“£»Fe-2e-ØTFe2+£»
£Ø3£©½šŹō±»øÆŹ“æģĀżĖ³ŠņĪŖ£ŗ×÷µē½ā³ŲŃō¼«£¾×÷Ōµē³Ųøŗ¼«£¾»ÆѧøÆŹ“£¾×÷Ōµē³ŲÕż¼«£¾×÷µē½ā³ŲŅõ¼«£¬£Ø1£©ÖŠĢś·¢Éś»ÆѧøÆŹ“”¢£Ø2£©ÖŠFe»īĘĆŠŌ“óÓŚSn¶ų×÷Ōµē³Ųøŗ¼«”¢£Ø3£©ÖŠFeµÄ»ī¶ÆŠŌŠ”ÓŚZn¶ų×÷Ōµē³ŲÕż¼«”¢£Ø4£©ÖŠFe×÷µē½ā³ŲŃō¼«£¬ĖłŅŌFeøÆŹ“æģĀżĖ³ŠņŹĒ£Ø4£©£¾£Ø2£©£¾£Ø1£©£¾£Ø3£©£¬¹Ź“š°øĪŖ£ŗ£Ø4£©£¾£Ø2£©£¾£Ø1£©£¾£Ø3£©£®
µćĘĄ ±¾Ģāæ¼²éøÖĢśµÄµē»ÆѧøÆŹ“ŅŌ¼°·Ą»¤£¬×¢ŅāĖįŠŌĢõ¼žĻĀøÖĢś·¢ÉśĪöĒāøÆŹ“£¬ČõĖįŠŌ»ņÖŠŠŌĢõ¼žĻĀøÖĢś·¢ÉśĪüŃõøÆŹ“£¬Ć÷Č·½šŹōøÆŹ“æģĀżĖ³ŠņŹĒ½ā±¾Ģā¹Ų¼ü£¬ÖŖµĄŌµē³ŲŗĶµē½ā³Ų×°ÖĆÖŠĢśĖł×÷µē¼«Ćū³Ę¼“æɽā“š£¬ĢāÄæÄŃ¶Č²»“ó£®


 Č«ÄÜĮ·æ¼¾ķĻµĮŠ“š°ø
Č«ÄÜĮ·æ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 16 | B£® | 14 | C£® | 12 | D£® | 28 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH4+2O2$\stackrel{µćČ¼}{”ś}$CO2+2H2O | |
| B£® | H2C=CH2+Br2”śCH3CHBr | |
| C£® |  | |
| D£® | CH3COOH+CH3CH2OH$”ś_{”÷}^{ÅØĮņĖį}$CH3CH2CH3+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
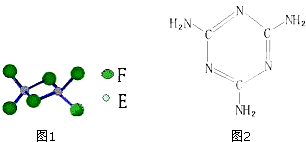
 ĘųĢåAŹĒŹÆÓĶĮŃ½āµÄÖ÷ŅŖ²śĪļÖ®Ņ»£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ28£¬CŹĒŅ»ÖŠĘųĢ壬EŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ£®A”¢B”¢C”¢D”¢EŌŚŅ»¶ØĢõ¼žĻĀ“ęŌŚČēĶ¼×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Ģõ¼ž”¢²śĪļ±»Ź”ĀŌ£©
ĘųĢåAŹĒŹÆÓĶĮŃ½āµÄÖ÷ŅŖ²śĪļÖ®Ņ»£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ28£¬CŹĒŅ»ÖŠĘųĢ壬EŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ£®A”¢B”¢C”¢D”¢EŌŚŅ»¶ØĢõ¼žĻĀ“ęŌŚČēĶ¼×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Ģõ¼ž”¢²śĪļ±»Ź”ĀŌ£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £Ø1£©ŗĻ³É°±¹¤Ņµ¶Ō¹śĆń¾¼ĆŗĶÉē»į·¢Õ¹¾ßÓŠÖŲŅŖµÄ ŅāŅ壮ijŗĻ³É°±¹¤ŅµÖŠĒāĘųÓÉĢģČ»ĘųŗĶĖ®·“Ó¦Öʱø£¬ĘäÖ÷ŅŖ·“Ó¦ĪŖ£ŗCH4£Øg£©+2H2O £Øg£©?CO2£Øg£©+4H2£Øg£©·“Ó¦¹ż³ĢÖŠÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņøĆ·“Ó¦ĪŖĪüČČ·“Ó¦£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©
£Ø1£©ŗĻ³É°±¹¤Ņµ¶Ō¹śĆń¾¼ĆŗĶÉē»į·¢Õ¹¾ßÓŠÖŲŅŖµÄ ŅāŅ壮ijŗĻ³É°±¹¤ŅµÖŠĒāĘųÓÉĢģČ»ĘųŗĶĖ®·“Ó¦Öʱø£¬ĘäÖ÷ŅŖ·“Ó¦ĪŖ£ŗCH4£Øg£©+2H2O £Øg£©?CO2£Øg£©+4H2£Øg£©·“Ó¦¹ż³ĢÖŠÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņøĆ·“Ó¦ĪŖĪüČČ·“Ó¦£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 =CHCOOCH2CH3£©µÄĀ·ĻßČēĻĀ£ŗ
=CHCOOCH2CH3£©µÄĀ·ĻßČēĻĀ£ŗ



 £©Óė»ÆŗĻĪļDCH3COOCH2CH3·¢Éś·“Ó¦£¬æÉÖ±½ÓŗĻ³ÉÓŠ»śĪļQ£®ŌņDµÄ½į¹¹¼ņŹ½ĪŖCH3COOCH2CH3£¬»ÆŗĻĪļCĆū³ĘĪŖ±½¼×Č©£®
£©Óė»ÆŗĻĪļDCH3COOCH2CH3·¢Éś·“Ó¦£¬æÉÖ±½ÓŗĻ³ÉÓŠ»śĪļQ£®ŌņDµÄ½į¹¹¼ņŹ½ĪŖCH3COOCH2CH3£¬»ÆŗĻĪļCĆū³ĘĪŖ±½¼×Č©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com