
��֪��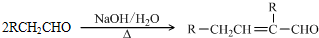
ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�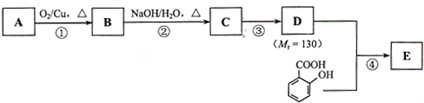
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ ���ṹ������ʾAֻ��һ������A������Ϊ ��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�� �ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ� ��
��4���ڢ۵ķ�Ӧ����Ϊ ��D���������ŵ�����Ϊ ��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ�� ��
A�������к���6��̼ԭ����һ������
B�����������������Ű���ˮ������еĹ�����
��6���ڢܲ��ķ�Ӧ����Ϊ ��д��E�Ľṹ��ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣�5-��-2,3-����-1-��ͪ�Ǻϳ���ũҩ���������Ҫ�м��塣
��֪��
�Ի�����A������ʽΪC7H7Cl��Ϊԭ�Ϻϳ�5-��-2,3-����-1-��ͪ��������F�������������£�
��д����ӦA��B�Ļ�ѧ����ʽ�� ��
�ƻ�����F�к��������ŵ�����Ϊ ����ӦB��C������Ϊ ��
��ij��������D��ͬ���칹�壬��ʹFeCl3��Һ����ɫ���ҷ�����ֻ��3�ֲ�ͬ��ѧ�������⡣д���û�����Ľṹ��ʽ�� (��дһ��)��
��E��F��ת���У������һ����F��Ϊͬ���칹��ĸ������ṹ��ʽΪ ��
�ɸ�������֪ʶ����������Ϣ��д���Ի�����F��CH2(COOC2H5)2Ϊ�л���Ӧԭ���Ʊ� �ĺϳ�·������ͼ(ע����Ӧ����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(ע����Ӧ����)���ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��6�֣���֪��A������ʯ�͵���Ҫ�л�����ԭ�ϣ�E�Ǿ��й���ζ���л��F��һ�ָ߾�����Ƴ�ʳƷ��װ���ϡ�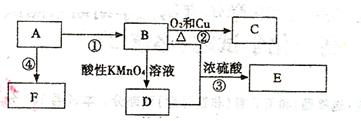
��1��A�Ľṹ��ʽΪ ��
��2��D�����еĹ������� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ��� 
��1����ҵ�ϣ���ʯ�ͻ��ʯ���͵ķ�����______����ʯ���ͻ��A��___ __�仯������������ѧ������
��2��д����ӦB+C�� D�Ļ�ѧ����ʽ�� _____________________________ __��
��3����������ʯ���ͻ��A�Ĺ����е��м����֮һ��д����������ͬ���칹��Ľṹ��ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���л��ﻯѧ������
A-G��Ϊ�л����������AΪ��±��������Է�������Ϊ216��̼����������Ϊ22.2%�����ת����ϵ���£�
��֪��
��ش�
��1��C�ĺ˴Ź���������_______�����շ塣
��2���١��ܵķ�Ӧ���ͷֱ�Ϊ________��______��
��3�����й���F��˵����ȷ����_______ (��ѡ����ĸ����
a��1 mol F�����������Ʒ�Ӧ�������2 mol H2
b��1 mol F��ȫȼ������8.5 mol O2
c����������Cu(OH)2��Ӧ����ש��ɫ����
d������NaHCO3��Ӧ����CO2
��4��д�����з�Ӧ�Ļ�ѧ����ʽ
��Ӧ��___________________________________��
��Ӧ��___________________________________��
��5����������������ͬ���칹����_____�֣���д����һ�ֵĽṹ��ʽ_____��
i����E��Ϊͬϵ�� ii����Է���������E��28
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��֪��ϡ��Һ��±����ˮ�⡣�����廯����C�ķ���ʽΪC9H9OCl��C�������ж���������������һ�ȴ���ֻ�ж��֣���˴Ź�������ͼ����������շ壬���շ�����֮��Ϊ1��2��2��2��2����һ�������£�������C�ɷ�����ͼ��ʾ��ת����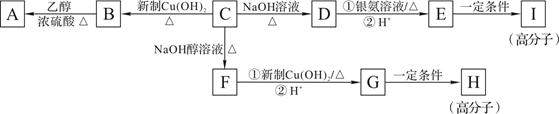
��1��B��A�ķ�Ӧ������_______��H�Ľṹ��ʽ��________��
��2��C������������_____��̼ԭ�ӹ��棬�京�������ŵ����� ��
��3��д�����л�ѧ����ʽ��D��������Һ��Ӧ_________��E��I________��
��4��D��һ��ͬϵ��W����ʽΪC8H8O2�����������������W��ͬ���칹�干��_____�֡���������
���ڷ����廯�������FeCl3��Һ������ɫ ��1 mol W���뺬2 mol NaOH����Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(13��) �ױ���һ����Ҫ�Ļ���ԭ�ϡ��Լױ�Ϊԭ��ͨ������ת���ɵö��ֻ�����Ʒ��
��֪��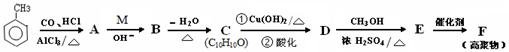
��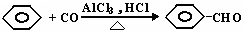
��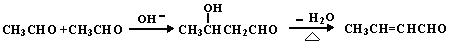
III�� A �б����ϵ�һ�ȴ���ֻ��2��
��1��д��ָ�����ʵĽṹ��ʽ��A ��M ��
��2��C�б����������������ŵ������� ��
��3��E��F�ķ�Ӧ������ ��
��4������ B ��˵����ȷ���� �������ţ�
| A���ܷ���������Ӧ | B����FeCl3��Һ������ɫ��Ӧ |
| C���ܷ�����ȥ��Ӧ | D������H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л���A�����µ�ת����ϵ:
��1��A�ķ���ʽΪ ��1molA�����Ժ� molH2��Ӧ��
��2��������C�Ľṹ��ʽΪ ���京�������ŵ������� ��
��3��A����������Ӧ�Ļ�ѧ����ʽΪ ��
��4������˵����ȷ���� ������ĸ����
a����Ӧ�ٵķ�Ӧ����Ϊ�ӳɷ�Ӧ
b��A��һ�ȴ�����2��
c��B�ܸ�Na��Ӧ�ų�H2������FeCl3��Һ����ɫ
d��1molD������NaOH��Һ��Ӧ��������2molNaOH
��5��C��ŨH2SO4�ͼ��ȵ������£���������D�⣬��������һ�ָ߷��ӻ�����E�� E�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣� ����mgij���壬����˫ԭ�ӷ��ӹ��ɣ�����Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ����
��1������������ʵ���Ϊ________mol��
��2������������ԭ������Ϊ_______ _����
��3���������ڱ�״���µ����Ϊ____________L��
��4������������1Lˮ�У������Ƿ�Ӧ��������Һ�����ʵ���������Ϊ
��5������������ˮ���γ�VL��Һ������Һ�����ʵ���Ũ��Ϊ_____ g��mol-1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com