
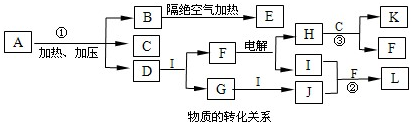
��15�֣�A��G�����л�����ǵ�ת����ϵ���£�
��ش��������⣺
����֪��6.0g������E��ȫȼ������8.8gCO2��3.6gH2O��E������������������ܶ�Ϊ30����E�ķ���ʽΪ ��
��AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ ��
����B����D����C����D�ķ�Ӧ�����ֱ��� �� ��
����A����B����D����G�ķ�Ӧ���ͷֱ��� �� ��
��F���������������У���ṹ��ʽΪ ��
����G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ��� �������к˴Ź�������������壬�ҷ������Ϊ1:1���� ����ṹ��ʽ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


 NH4++NH2-
NH4++NH2- NH4++NH2-
NH4++NH2-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?����һģ��ʵ����A��B��C�����л������Է���������Ϊ72��
��2008?����һģ��ʵ����A��B��C�����л������Է���������Ϊ72�� +nCH3OH
+nCH3OH| ���� |
| ���� |
 +nH2O
+nH2O +nCH3OH
+nCH3OH| ���� |
| ���� |
 +nH2O
+nH2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������CO��HCOOH��HOOC��CHO����ȩ�ᣩ�ֱ�ȼ��ʱ�����ĵ����������ɵĶ�����̼������ȶ���1��2�������ߵķ���ʽ���Էֱ��ǣ�CO����H2O���ͣ�CO��2��H2O����Ҳ����˵��ֻҪ����ʽ���ϣ�CO��n(H2O)m�� n��m��Ϊ���������ĸ����л������ȼ��ʱ���ĵ����������ɵĶ�����̼�����������1��2��
����һЩֻ��C��H��O����Ԫ�ص��л������ȼ��ʱ���ĵ����������ɵĶ�����̼���������3��4��
��1����Щ�л����У���Է���������С�Ļ�������________��
��2��ij����̼ԭ������ͬ�������л�������ǵ���Է��������ֱ�Ϊa��b��a��b������a-b�ض���________������һ�����֣�����������
��3������Щ�л�������һ�ֻ�����������������ǻ���ȡ0.2625 g���л���ǡ���ܸ�25.00 mL 0.100 mol��L-1 NaOH��Һ��ȫ�кͣ��ɴ˿��Լ����֪�û��������Է���������__________________�������Ƶ����ķ���ʽӦΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ��Ϫһ�и߶���ѧ�����п��ԣ�������ѧ�Ծ� ���������� ���ͣ���ѡ��
�л���Ľṹ��ʽ���£�����ͨ����ͬ�Ļ�ѧ��Ӧ�ֱ��Ƶýṹ��ʽΪA~G������
��ش��������⣺
��С��1��ָ����Ӧ�����ͣ�A��C�� A��D�� ��
��С��2��A-G�пɿ���������� ��(����ţ���ͬ)
��С��3����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ�������������������ԭ���п��ܶ���ͬһƽ���ڵ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com