
K2Cr2O7ŗĶH2O2ŌŚH2SO4ČÜŅŗÖŠ»ģŗĻŹ±£¬æɹŪ²ģµ½ĻĀĆęĮ½øöĻÖĻó£ŗ
¢Ł»ģŗĻŗó5”«10ĆėÄŚ£¬ČÜŅŗÓɳČÉ«±äĪŖ°µĄ¶É«£»
¢ŚŌŚ80”«150ĆėÄŚÓÉ°µĄ¶É«±äĪŖĀĢÉ«£¬Óė“ĖĶ¬Ź±·Å³öĘųÅŻ”£ÓėÖ®ĻąÓ¦µÄĮ½øö·“Ó¦ĪŖ£ŗ
A£®Cr2O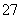 £«H2O2£«H£«ØD”śCrO5£«H2O
£«H2O2£«H£«ØD”śCrO5£«H2O
B£®CrO5£«H£«ØD”śCr3£«£«O2”ü£«H2O
(1)ŅŃÖŖ·“Ó¦A²»ŹĒŃõ»Æ»¹Ō·“Ó¦£¬ŌņCrO5ÖŠCrŌŖĖŲµÄ¼ŪĢ¬ĪŖ____________”£
(2)·“Ó¦BŹĒ·ńŹōÓŚŃõ»Æ»¹Ō·“Ó¦£æ
“š£ŗ____________£¬ĘäĄķÓÉŹĒ____________________________________________”£
(3)½«·“Ó¦AŗĶ·“Ó¦BĻą¼Ó£¬æÉŅŌµĆµ½Ņ»øö×Ü»Æѧ·½³ĢŹ½£¬øĆ×Ü·“Ó¦ÖŠŃõ»Æ¼ĮŹĒ________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
25”ꏱ£¬ŃĪĖįČÜŅŗµÄpHŹĒ4£¬ĀČ»Æļ§ČÜŅŗµÄpHŹĒ5£¬ŃĪĖįÓėĀČ»Æļ§ČÜŅŗÖŠĖ®µēĄėµÄc£ØH+£©Ö®±ČĪŖ£Ø £©
A£®10£ŗ1 B£®1£ŗ10 C£®1£ŗ105 D£® 105£ŗ1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾£¬ŌŚŹ¢ÓŠĖ®µÄÉÕ±ÖŠ£¬ĢśČ¦ŗĶŅųȦµÄĮ¬½Ó“¦µõÓŠŅ»øł¾ųŌµµÄĻøĖ棬Ź¹Ö®Ę½ŗā”£
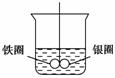
(1)ĻņÉÕ±ÖŠ¼ÓČėCuSO4ČÜŅŗ£¬Ę¬æĢŗóæɹŪ²ģµ½µÄĻÖĻó(ÖøŠüµõµÄ½šŹōȦ)ŹĒ(””””)
A£®ĢśČ¦ŗĶŅųȦ×óÓŅŅ”°Ś²»¶Ø
B£®±£³ÖĘ½ŗāדĢ¬²»±ä
C£®ĢśČ¦ĻņĻĀĒ抱
D£®ŅųȦĻņĻĀĒ抱
(2)²śÉśÉĻŹöĻÖĻóµÄŌŅņŹĒ”¢________________________________________________
________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃĒĻõĖį(HNO2)ŌŚ·“Ó¦ÖŠ¼ČæÉ×öŃõ»Æ¼Į£¬ÓÖæÉ×ö»¹Ō¼Į”£µ±Ėü×ö»¹Ō¼ĮŹ±£¬ĘäŃõ»Æ²śĪļæÉÄÜŹĒ(””””)
A£®NH3 B£®N2 C£®NO2 D£®NO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚH£«”¢Mg2£«”¢Fe2£«”¢S2£”¢I£”¢SÖŠ£¬Ö»¾ßÓŠŃõ»ÆŠŌµÄŹĒ______________________£¬Ö»¾ßÓŠ»¹ŌŠŌµÄŹĒ________________£¬¼ČÓŠŃõ»ÆŠŌÓÖÓŠ»¹ŌŠŌµÄŹĒ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
×ćĮæµÄÅØH2SO4ŗĶm gĶĶźČ«·“Ó¦£¬µĆµ½±ź×¼×“æöĻĀn L SO2£¬Ōņ±»»¹ŌµÄĮņĖįŹĒ(””””)
A.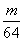 mol B.
mol B.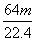 mol
mol
C.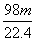 g D.
g D.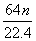 g
g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĖįÓźŹĒÖøĢģæÕ½µĖ®³ŹĖįŠŌ(pHŠ”ÓŚ5.6)£¬øö±šµŲ·½ĖįÓźµÄpH¾¹µĶÓŚ2.1(Ź³“×µÄpH£½3)”£ŠĪ³ÉĖįÓźµÄÖ÷ŅŖŹĒ“óĘųÖŠµÄSO2ŗĶµŖŃõ»ÆĪļ”£ĖüĆĒµÄÖ÷ŅŖĄ“Ō“ŹĒĆŗŗĶŹÆÓĶµÄČ¼ÉÕ£¬Č«ŹĄ½ēĆæÄźÓŠ1.5ŅŚ¶ÖSO2µÄÅÅ·ÅĮ攣
(1)SO2æÉŌŚæÕĘųÖŠŹÜ¹āÕÕ±»Ńõ»Æ£¬×īÖÕÓėÓźĖ®ŠĪ³ÉĖįÓź”£ŹŌŠ“³öÕāĮ½øö»Æѧ·“Ó¦·½³ĢŹ½£ŗ
________________________________________________________________________
________________________________________________________________________ӣ
(2)Ęū³µÅŷŵÄĪ²Ęų”¢ĻõĖį³§ŗĶ»Æ·Ź³§µÄ·ĻĘų¶¼ŗ¬µŖŃõ»ÆĪļ£¬Č«ŹĄ½ēĆæÄźÅÅ·ÅĮæŌ¼5”Į107 kg”£NO2ČÜÓŚĖ®Éś³É________Ėį”£
(3)ĖįÓźæɵ¼ÖĀµÄĪ£ŗ¦ÓŠ(””””)
A£®øÆŹ“½ØÖžĪļ B£®µ¼ÖĀŹ÷ľæŻĪ®
C£®Ōģ³ÉŗéĄŌŌÖŗ¦ D£®¶ń»ÆČĖĄą»·¾³
(4)ĪŖĮĖ¼õÉŁĖįÓźµÄŠĪ³É£¬±ŲŠė¼õÉŁSO2µÄÅÅ·ÅĮ棬¶ŌČ¼ĮĻÖŠµÄĮņ»ÆĪļ½ųŠŠ________£¬¶Ō·ĻĘųÖŠµÄµŖŃõ»ÆĪļ½ųŠŠ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³żČ„ĻĀĮŠĪļÖŹÖŠĖłŗ¬µÄŌÓÖŹ(ĄØŗÅÄŚĪŖŌÓÖŹ)£¬½«Ń”ÓƵďŌ¼ĮŗĶ·ÖĄė·½·ØĢīŌŚĢāŗóµÄŗįĻßÉĻ£¬²¢Š“³öÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½”£
(1)Fe2O3[Fe(OH)3]______________________________£»
(2)Fe2O3(Al2O3)_________________________________________________________£»
(3)FeCl3(FeCl2)__________________________________________________________£»
(4)FeCl2(FeCl3)___________________________________________________________£»
(5)FeCl2(CuCl2)___________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŅĒĘ÷ÄÜÓĆ¾Ę¾«µĘÖ±½Ó¼ÓČȵďĒ
A.ÉÕĘæ B.ČŻĮæĘæ C.ŹŌ¹Ü D.ÉÕĘæ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com