
·ÖĪö £Ø1£©ÅäÖĘ2.5mol/L µÄĻ”ĮņĖįČÜŅŗ90mL£¬Ó¦Ń”Ōń100mLČŻĮæĘ棬ŅĄ¾ŻC=$\frac{1000¦Ń¦Ų}{M}$¼ĘĖćÖŹĮæ·ÖŹżĪŖ98%£¬ĆܶČĪŖ1.84g/cm3µÄÅØĮņĖįµÄĪļÖŹµÄĮæÅØ¶Č£¬ŅĄ¾ŻČÜŅŗĻ”ŹĶ¹ż³ĢÖŠČÜÖŹŹĒĪļÖŹµÄĮæ±£³Ö²»±ä¼ĘĖćŠčŅŖÅØĮņĖįµÄĢå»ż£»
£Ø2£©ÅäÖĘĻ”ĮņĖįČÜŅŗĻ”ŹĶŹ±ŠčŅŖÓĆ²£Į§°ō½Į°č£¬ŅĘŅŗŹ±ŠčŅŖÓĆ²£Į§°ōŅżĮ÷£»
£Ø3£©øł¾Żc=$\frac{n}{V}$æÉµĆ£¬Ņ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗÅäÖʵÄĪó²ī¶¼ŹĒÓÉČÜÖŹµÄĪļÖŹµÄĮænŗĶČÜŅŗµÄĢå»żVŅżĘšµÄ£¬Īó²ī·ÖĪöŹ±£¬¹Ų¼üŅŖæ“ÅäÖĘ¹ż³ĢÖŠŅżĘšnŗĶVŌõŃłµÄ±ä»Æ£»Čōn±ČĄķĀŪÖµŠ”£¬»ņV±ČĄķĀŪÖµ“óŹ±£¬¶¼»įŹ¹ĖłÅäČÜŅŗÅضČĘ«Š”£»Čōn±ČĄķĀŪÖµ“󣬻ņV±ČĄķĀŪÖµŠ”Ź±£¬¶¼»įŹ¹ĖłÅäČÜŅŗÅضČĘ«“ó£»
£Ø4£©øł¾Żn=cV¼ĘĖć³öĮņĖįµÄĪļÖŹµÄĮ棬ŌŁ¼ĘĖć³öŗ¬ÓŠĒāĄė×ÓµÄĪļÖŹµÄĮ棬“Ó¶ųæÉÖŖĻūŗÄĒāŃõ»ÆÄʵÄĪļÖŹµÄĮ棬ŌŁøł¾ŻV=$\frac{n}{C}$¼ĘĖć³öĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»ż£»øł¾Żc=$\frac{n}{V}$¼ĘĖć³öÄĘĄė×ÓµÄÅØ¶Č£®
½ā“š ½ā£ŗ£Ø1£©ŹµŃéŹŅĪŽ90mlČŻĮæĘ棬Šė°“100mlČŻĮæĘæ¼ĘĖć£¬øł¾ŻCÅØ”ĮVÅØ=CĻ””ĮVĻ”£¬2.5mol/L”Į0.1L=$\frac{VmL”Į1.84g/c{m}^{3}”Į98%}{98g/mol}$£¬½āµĆV=13.6mL£¬
¹Ź“š°øĪŖ£ŗ13.6£»
£Ø2£©ÅäÖĘĻ”ĮņĖįČÜŅŗĻ”ŹĶŹ±ŠčŅŖÓĆ²£Į§°ō½Į°č£¬ŅĘŅŗŹ±ŠčŅŖÓĆ²£Į§°ōŅżĮ÷£»
¹Ź“š°øĪŖ£ŗ½Į°č”¢ŅżĮ÷£»
£Ø3£©A£®ĮæČ”ÅØĮņĖįŹ±£¬ŃöŹÓ¶ĮŹż£¬µ¼ÖĀĮæČ”µÄÅØĮņĖįĢå»żĘ«“ó£¬ČÜÖŹµÄĪļÖŹµÄĮæĘ«“ó£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹAŃ”£»
B£®Ļ“µÓĮæČ”ÅØH2SO4ŗóµÄĮæĶ²£¬²¢½«Ļ“µÓŅŗ×ŖŅʵ½ČŻĮæĘæÖŠ£¬µ¼ÖĀĮæČ”µÄÅØĮņĖįĢå»żĘ«“ó£¬ČÜÖŹµÄĪļÖŹµÄĮæĘ«“ó£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹBŃ”£»
C£®Ļ”ŹĶĮņĖįŹ±£¬ÓŠČÜŅŗ½¦µ½×ĄĆęÉĻ£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹC²»Ń”£»
D£®Ć»ÓŠĻ“µÓĻ”ŹĶĮņĖįµÄÉÕ±ŗĶ²£Į§°ō£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹC²»Ń”£»
E£®¶ØČŻŅ”ŌČŗ󣬷¢ĻÖŅŗĆęµĶÓŚ±źĻߣ¬ÓÖÓĆ½ŗĶ·µĪ¹Ü¼ÓÕōĮóĖ®ÖĮ±źĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹD²»Ń”£»
F£®ČŻĮæĘæ²»øÉŌļ£¬¶ŌČÜÖŹŹĒĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»»į²śÉśÓ°ĻģČÜŅŗÅØ¶Č²»±ä£¬¹ŹF²»Ń”£»
¹ŹŃ”£ŗA B£»
£Ø4£©40mL 2.5mol/LµÄĻ”ĮņĖįÖŠŗ¬ÓŠĒāĄė×ÓµÄĪļÖŹµÄĮæĪŖ£ŗ2.5mol/L”Į2”Į0.04L=0.2mol£¬·¢ÉśÖŠŗĶ·“Ó¦ŠčŅŖĻūŗÄ0.2molNaOH£¬ĻūŗÄ5mol/LµÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żĪŖ£ŗ$\frac{0.2mol/L}{5mol/L}$=0.04L=40mL£»
»ģŗĻŅŗĢå»żĪŖ40mL+40mL=80mL=0.08L£¬»ģŗĻŅŗÖŠŗ¬ÓŠÄĘĄė×ÓµÄĪļÖŹµÄĮæĪŖ0.2mol£¬Ōņ·“Ó¦ŗóÄĘĄė×ÓÅضČĪŖ£ŗ$\frac{0.2mol}{0.08L}$=2.5mo/L£¬
¹Ź“š°øĪŖ£ŗ40£»2.5molØML£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ·½·Ø£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ·½·ØĪŖ½ā“š¹Ų¼ü£¬×¢ŅāÕĘĪÕĪó²ī·ÖĪöµÄ·½·ØÓė¼¼ĒÉ£¬ŹŌĢāÅąŃųĮĖѧɜµÄѧɜµÄ»ÆѧŹµŃéÄÜĮ¦£®


 ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
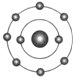 ŅŃÖŖŅ»°ćĒéæöĻĀŌ×ÓŗĖĶā×īĶā²ćµē×ÓŹżĻąµČµÄŌŖĖŲ¾ßÓŠĻąĖʵĻÆѧŠŌÖŹ£®·śŌŖĖŲŌ×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź¾ŅāĶ¼ĪŖ£®ĻĀĮŠŌ×ÓÖŠ£¬Óė·śŌŖĖŲŌ×ӵĻÆѧŠŌÖŹĻąĖʵďĒ£Ø””””£©
ŅŃÖŖŅ»°ćĒéæöĻĀŌ×ÓŗĖĶā×īĶā²ćµē×ÓŹżĻąµČµÄŌŖĖŲ¾ßÓŠĻąĖʵĻÆѧŠŌÖŹ£®·śŌŖĖŲŌ×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź¾ŅāĶ¼ĪŖ£®ĻĀĮŠŌ×ÓÖŠ£¬Óė·śŌŖĖŲŌ×ӵĻÆѧŠŌÖŹĻąĖʵďĒ£Ø””””£©| A£® | 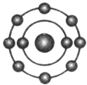 | B£® | 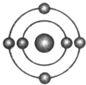 | C£® | 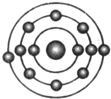 | D£® | 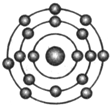 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŻĶČ”·ÖŅŗ | B£® | ŹÆÓĶµÄ·ÖĮó | C£® | Õō·¢½į¾§ | D£® | øÖĢśøÆŹ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HOCH2CH2OH | B£® | HOOC-CH2CH2-OH | C£® | HOOC-COOH | D£® | CH3COOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{1}{28}$m/mol | B£® | $\frac{1}{22.4}$m/mol | C£® | 22.4m/mol | D£® | 22.4 m/mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ėį»ÆµÄµķ·ŪČÜŅŗ | B£® | ĮņĖįÄĘČÜŅŗ | C£® | H2O2ČÜŅŗ | D£® | ĀČĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1 L 0.1 mol•L-1µÄ°±Ė®ÖŠŗ¬ÓŠNH3•H2O·Ö×ÓŹżĪŖ0.1NA | |
| B£® | µē½ā¾«Į¶ĶŹ±£¬ČōŃō¼«ÖŹĮæ¼õÉŁ6.4 g£¬ŌņµēĀ·ÖŠ×ŖŅʵē×ÓŹżĪŖ0.2NA | |
| C£® | ±ź×¼×“æöĻĀ£¬2.24 LµÄ¶žĀČ¼×ĶéÖŠŗ¬ÓŠµÄĢ¼ĀČ¹²¼Ū¼üµÄŹżÄæĪŖ0.2NA | |
| D£® | Ņ»¶ØĢõ¼žĻĀ£¬4.6 g NO2ŗĶN2O4»ģŗĻĘųĢåÖŠŗ¬ÓŠµÄNŌ×ÓŹżÄæĪŖ0.1NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ķ¬Ņ»ŌŖĖŲ²»æÉÄÜ¼Č±ķĻÖ½šŹōŠŌ£¬ÓÖ±ķĻÖ·Ē½šŹōŠŌ | |
| B£® | Ķ¬Ņ»Ö÷×åµÄŌŖĖŲµÄŌ×Ó£¬×īĶā²ćµē×ÓŹżĻąĶ¬£¬»ÆѧŠŌÖŹĶźČ«ĻąĶ¬ | |
| C£® | ¶ĢÖÜĘŚŌŖĖŲŠĪ³ÉĄė×Óŗó£¬×īĶā²ć¶¼“ļµ½8µē×ÓĪČ¶Ø½į¹¹ | |
| D£® | µŚČżÖÜĘŚÖ÷×åŌŖĖŲµÄ×īøßÕż»ÆŗĻ¼ŪµČÓŚĖüĖł“¦µÄÖ÷×åŠņŹż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com