
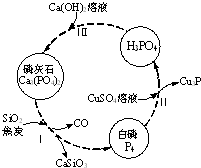
 Į×¼°²æ·ÖÖŲŅŖ»ÆŗĻĪļµÄĻą»„×Ŗ»ÆČēĶ¼ĖłŹ¾£®
Į×¼°²æ·ÖÖŲŅŖ»ÆŗĻĪļµÄĻą»„×Ŗ»ÆČēĶ¼ĖłŹ¾£®| ³É·Ö | CaO | P2O5 | SO3 | CO2 |
| ÖŹĮæ·ÖŹż£Ø%£© | 47.30 | 28.40 | 3.50 | 6.10 |
·ÖĪö £Ø1£©ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦ÖŠĶŌŖĖŲ”¢Į×ŌŖĖŲ»ÆŗĻ¼Ū±ä»Æ¼ĘĖć£»
£Ø2£©Į×ĖįĪŖČżŌŖĖįÓėĒāŃõ»ÆøĘ·“Ó¦£¬ŅĄ¾ŻĮ×ĖįÓėĒāŃõ»ÆøĘĮæ²»Ķ¬£¬µĆµ½µÄ²śĪļæÉÄÜŹĒ£ŗCa3£ØPO4£©2”¢CaHPO4”¢Ca£ØH2PO4£©2£»
£Ø3£©Į×»ŅŹÆÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹż=¶žŃõ»ÆĢ¼ÖŹĮæ·ÖŹż”Į¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲÖŹĮæ·ÖŹż£»
£Ø4£©øł¾ŻøĘŌŖĖŲŹŲŗć¼ĘĖć£»
£Ø5£©ÓĆm±ķŹ¾³öĮ×»ŅŹÆÖŠCa”¢S”¢PŌŖĖŲĪļÖŹµÄĮ棬¼ĘĖć³öĮ×ĖįÖŠPŌŖĖŲĪļÖŹµÄĮ攢ĮņĖįÖŠSŌŖĖŲĪļÖŹµÄĮ棬½įŗĻ»ÆѧŹ½æÉÖŖn£ØCa£©=n£ØS£©+$\frac{1}{2}$n£ØP£©£¬¾Ż“ĖĮŠ·½³Ģ½ā“š£®
½ā“š ½ā£ŗ£Ø1£©CuŌŖĖŲµÄ»ÆŗĻ¼ŪÓÉ+2¼Ū½µµĶµ½+1¼Ū£¬CuSO4ŹĒŃõ»Æ¼Į£¬P4²æ·ÖĮ×ŌŖĖŲÓÉ0¼Ū½µµĶµ½-3¼Ū£¬²æ·ÖĮ×ŌŖĖŲÓÉ0¼ŪÉżøßµ½+5¼Ū£¬Į×ŌŖĖŲµÄ»ÆŗĻ¼Ū¼ČÉżøßÓÖ½µµĶ£¬ĖłŅŌP4¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į£¬ČōÓŠ11molP4²Ī¼Ó·“Ó¦£¬ĘäÖŠ5molµÄP4×öŃõ»Æ¼Į£¬60molĮņĖįĶ×öŃõ»Æ¼Į£¬Ö»ÓŠ6molµÄP4×ö»¹Ō¼Į£¬ŌņÓɵē×ÓŹŲŗćæÉÖŖ£¬ÓŠ1 molµÄCuSO4²Ī¼Ó·“Ó¦£¬Ōņ±»ĮņĖįĶŃõ»ÆµÄ°×Į×·Ö×ÓµÄĪļÖŹµÄĮæĪŖnŌņ£ŗn”Į4£Ø5-0£©=1mol”Į£Ø2-1£©£¬½āµĆn=0.05mol£»
¹Ź“š°øĪŖ£ŗ0.05mol
£Ø2£©Į×ĖįĪŖČżŌŖĖįÓėĒāŃõ»ÆøĘ·“Ó¦£¬ŅĄ¾ŻĮ×ĖįÓėĒāŃõ»ÆøĘĮæ²»Ķ¬£¬µĆµ½µÄ²śĪļæÉÄÜŹĒ£ŗCa3£ØPO4£©2”¢CaHPO4”¢Ca£ØH2PO4£©2£»
¹Ź“š°øĪŖ£ŗCaHPO4”¢Ca£ØH2PO4£©2£»
£Ø1£©Į×»ŅŹÆÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹż=6.10%”Į$\frac{12}{44}$=1.66%£¬¹Ź“š°øĪŖ£ŗ1.66%£»
£Ø2£©100gĮ×»ŅŹÆ·ŪÄ©ÖŠCaŌŖĖŲÖŹĮæ=100g”Į47.3%”Į$\frac{40}{56}$£¬øĘŌŖĖŲČ«²æŅŌCaSO4µÄŠĪŹ½“ęŌŚ£¬øł¾ŻCaŌŖĖŲŹŲŗćæÉÖŖ£¬æÉŅŌµĆµ½CaSO4µÄÖŹĮæ=$\frac{100g”Į47.3%”Į\frac{40}{56}}{\frac{40}{136}}$=114.87g£¬
¹Ź“š°øĪŖ£ŗ114.87£»
£Ø3£©mgĮ×»ŅŹÆÖŠCaŌŖĖŲĪļÖŹµÄĮæ=mg”Į47.3%”Ā56g/mol=0.00845m mol£¬SŌŖĖŲĪļÖŹµÄĮæ=mg”Į3.5%”Ā80g/mol=0.00044m mol£¬PŌŖĖŲĪļÖŹµÄĮæ=mg”Į28.4%”Į$\frac{62}{142}$”Ā31g/mol=0.004m mol£¬
Į×ĖįÖŠPŌŖĖŲĪļÖŹµÄĮæ=0.05L”Į0.5mol/L=0.025mol£¬ĮņĖįÖŠSŌŖĖŲÖŹĮæ=0.05L”Į0.1mol/L=0.005mol£¬
ÓÉ»ÆѧŹ½æÉÖŖ£¬n£ØCa£©=n£ØS£©+$\frac{1}{2}$n£ØP£©£¬¹Ź0.00845m=£Ø0.00044m+0.005£©+£Ø0.004m+0.025£©”Į$\frac{1}{2}$£¬
½āµĆm=2.91£¬
“š£ŗmµÄÖµĪŖ2.91£®
µćĘĄ ±¾Ģāæ¼²éÖŹĮæ·ÖŹż¼ĘĖć”¢»ģŗĻĪļ¼ĘĖćµČ£¬ĄūÓĆŹŲŗć½ųŠŠ¼ĘĖć£¬¹Ų¼üĆ÷Č·CaŌŖĖŲƻӊȫ²æĄūÓĆ£¬PŌŖĖŲČ«²æĄūÓĆ£¬ĢāÄæ¼ĘĖćĮæŗÜ“ó£¬ĢāÄæÄŃ¶Č½Ļ“ó£®


 ÅąÓÅČżŗĆÉśĻµĮŠ“š°ø
ÅąÓÅČżŗĆÉśĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓėĖ®·“Ó¦ | B£® | ÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦ | ||
| C£® | ÓėĮņĖį·“Ó¦ | D£® | ÓėĒ°ČżÖÖĪļÖŹ¾łÄÜ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹ā·ü·¢µēÖ÷ŅŖĄūÓĆøß“æ¶Čµ„ÖŹ¹čÖĘ³ÉµÄ¹čĢ«ŃōÄܵē³Ų | |
| B£® | ¹čĢ«ŃōÄܵē³Ųæɽ«Ģ«ŃōÄÜÖ±½Ó×Ŗ»ÆĪŖµēÄÜ£¬¼õÉŁ»ÆŹÆČ¼ĮĻµÄŹ¹ÓĆ£¬±£»¤»·¾³ | |
| C£® | ¹čµÄ½į¹¹ŗĶ½šøÕŹÆĄąĖĘ£¬ŹĒ¾ßÓŠ½šŹō¹āŌóµÄ»ŅŗŚÉ«¹ĢĢå | |
| D£® | ¹čµ„ÖŹ¼“ÄÜÓėĒāŃõ»ÆÄĘ·“Ó¦ÓÖÄÜÓėĒā·śĖį·“Ó¦£¬ĖłŅŌ¹čŹĒĮ½ŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÉĻŹöŹµŃéÖŠ²»ÄÜÓĆŃĪĖį“śĢęĮņĖį | |
| B£® | ½«ŹŌ¹Ü³ä·ÖÕńµ“ŗó¾²ÖĆ£¬ČÜŅŗŃÕÉ«±äĪŖ×ĻÉ« | |
| C£® | ŌŚĖįŠŌĢõ¼žĻĀ£¬PbO2µÄŃõ»ÆŠŌ±ČMnO4-µÄŃõ»ÆŠŌĒæ | |
| D£® | ČōĮņĖįĆĢ³ä·Ö·“Ó¦£¬ĻūŗÄPbO2µÄĪļÖŹµÄĮæĪŖ0.01mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®| I1 | I2 | I3 | I4 | ” | |
| µēĄėÄÜ£ØkJ/mol£© | 738 | 1451 | 7733 | 10540 | ” |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | 6.02”Į1023 | B£® | 12CŌ×ÓÖŹĮæµÄŹ®¶ž·ÖÖ®Ņ» | ||
| C£® | 0.012Kg12CĖłŗ¬µÄŌ×ÓŹż | D£® | 1molŃõĘųĖłŗ¬µÄĪ¢Į£Źż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²ā¶ØÕōĘųµÄĦ¶ūĢå»ż | |
| B£® | ÓėŅų°±ČÜŅŗ·“Ó¦£¬·ÖĪö²śÉśŅųµÄĮæ | |
| C£® | ÓėÄĘ·“Ó¦£¬·ÖĪö²śÉśĒāĘųµÄĮæ | |
| D£® | ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶Ø£¬·ÖĪöĻą¹ŲŹż¾Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
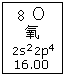
| A£® | ŃõŌŖĖŲµÄÖŹĮæŹżŹĒ16 | |
| B£® | ŃõŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæŹĒ16.00 | |
| C£® | ŃõŌ×Ó2pŃĒ²ćÓŠŅ»øöĪ“³É¶Ōµē×Ó | |
| D£® | ŃõŌ×Ó×īĶā²ćÓŠ6øöÄÜĮæĻąĶ¬µÄµē×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NO3- | B£® | SO42- | C£® | Na+ | D£® | H+ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com