
ؤ³»¯ر§ذثب¤ذ،×éہûسأؤ³·دئْµؤرُ»¯حذ؟؟َضئب،»îذشZnOتµرéء÷³جبçدآ£؛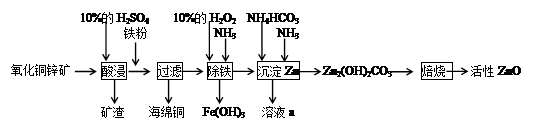
اë»ط´ًدآءذختجâ£؛
£¨1£©¼سبëجْ·غ؛َ·¢ةْ·´س¦µؤہë×س·½³جت½خھ_________________________________،£
£¨2£©¼×،¢ززء½ح¬ر§ر،سأدآءذزائ÷£¬²ةسأ²»ح¬µؤ·½·¨ضئب،°±ئّ،£
¢ظ¼×ح¬ر§ت¹سأµؤز©ئ·تاتىت¯»زسëآب»¯ï§£¬شٍس¦ر،سأ×°ضأ_______£¨جîذ´×°ضأ´ْ؛إ£©£¬ةْ³ة°±ئّµؤ»¯ر§·½³جت½خھ____________________________________________________________________________£»
¢عززح¬ر§ر،سأءث×°ضأB£¬شٍت¹سأµؤء½ضضز©ئ·µؤأû³ئخھ_______________،£
£¨3£©H2O2µؤ×÷سأتا____________________________________________________،£
£¨4£©³جْ¹³جضذµأµ½µؤFe(OH)3؟ةسأKClOبـز؛شع¼îذش»·¾³½«ئنرُ»¯µأµ½ز»ضض¸كذ§µؤ¶à¹¦ؤـث®´¦ہي¼ء£¨K2FeO4£©£¬¸أ·´س¦ضذرُ»¯¼ءس뻹ش¼ءµؤخïضتµؤء؟ض®±بخھ____________،£
£¨5£©زرضھبـز؛aضذ؛¬سذCO32£،¢SO42£ء½ضضثل¸ùزُہë×س£¬بôض»شتذيب،سأز»´خرùئ·£¬¼ىرéصâضضہë×س´وشعµؤتµرé²ظ×÷¹³جخھ_______________________________________________________________________،£
£¨1£©Fe£«2H£«£½Fe2£«£«H2،ü£¨1·ض£© Fe£«Cu2£«£½Fe2£«£«Cu£¨1·ض£©
£¨2£©¢ظA£¨1·ض£© 2NH4Cl£«Ca(OH)2 CaCl2£«2NH3£«2H2O(2·ض)
CaCl2£«2NH3£«2H2O(2·ض)
¢عإ¨°±ث®£¨1·ض£© ¼îت¯»ز»ٍةْت¯»ز»ٍاâرُ»¯ؤئ¹ججه£¨2·ض£©
£¨3£©½«بـز؛ضذµؤFe2£«رُ»¯³ةFe3£«£¨2·ض£© £¨4£©3:2£¨4·ض£©
£¨5£©ب،ةظء؟بـز؛aسعتش¹ـضذ£¬µخ¼سBaCl2»ٍBa(NO3)2بـز؛£¬¹آث£¬دٍثùµأ³ءµيضذ£¬µخ¼س¹ء؟µؤرخثل»ٍدُثل£¬³ءµي²؟·ضبـ½â£¬ثµأ÷سذCO32£،¢SO42£،£
½âخِتشجâ·ضخِ£؛£¨1£©ثل½؛َثùµأبـز؛تاءٍثلح،¢ءٍثلذ؟؛ح¹ء؟µؤءٍثل،£جْتا»îئأµؤ½ًتô£¬½ًتôذشا؟سعحµؤ£¬بُسعذ؟µؤ£¬ثùزش¼سبëجْ·غ؛َ·´س¦µؤہë×س·½³جت½خھFe£«2H£«£½Fe2£«£«H2،ü،¢Fe£«Cu2£«£½Fe2£«£«Cu،£
£¨2£©¢ظتىت¯»زسëآب»¯ï§¶¼تا¹ججهز©ئ·£¬²»ذèزھ·ضز؛آ©¶·£¬شٍس¦ر،سأ×°ضأتاA£¬·´س¦µؤ»¯ر§·½³جت½خھ2NH4Cl£«Ca(OH)2 CaCl2£«2NH3£«2H2O،£
CaCl2£«2NH3£«2H2O،£
¢ع¸ù¾فB×°ضأµؤجطµم؟ةضھ£¬¸أ×°ضأتتسأسع¹ججهسëز؛جه»ٍز؛جهسëز؛جهض®¼ن²»ذèزھ¼سببضئ±¸ئّجه£¬زٍ´ثبç¹ûسأ¸أ×°ضأضئ±¸°±ئّ£¬شٍ·ضز؛آ©¶·ضذت¢·إµؤتش¼ءتاإ¨°±ئّ£¬ةصئ؟ضذت¢·إµؤتا¼îت¯»ز»ٍةْت¯»ز»ٍاâرُ»¯ؤئ¹ججه،£ہûسأ¼îت¯»ز»ٍةْت¯»ز»ٍاâرُ»¯ؤئ¹ججهبـسعث®·إببازشِ´َبـز؛ضذOH£إ¨¶ب£¬´س¶ّ´ظ½ّ°±ث®ضذز»ث®؛د°±·ض½â¶ّ·إ³ِ°±ئّ،£
£¨3£©¹آث؛َبـز؛ضذ؛¬سذراجْہë×س£¬زھ×ھ»¯خھاâرُ»¯جْ³ءµي£¬ذèزھ½«راجْہë×سرُ»¯ةْ³ةجْہë×س£¬ثùزش¼سبëث«رُث®µؤ×÷سأتا½«بـز؛ضذµؤFe2£«رُ»¯³ةFe3£«،£
£¨4£©اâرُ»¯جْ±»´خآبثلؤئرُ»¯ةْ³ة¸كجْثل¼ط£¬ئنضذجْشھثطµؤ»¯؛د¼غ´س£«3¼غة¸كµ½£«6¼غ£¬ت§ب¥3¸ِµç×س،£´خآبثلؤئضذآبشھثطµؤ»¯؛د¼غ´س£«1¼غ½µµحµ½£1¼غ£¬µأµ½2¸ِµç×س،£زٍ´ث¸ù¾فµç×سµأت§تط؛م؟ةضھ£¬·´س¦ضذرُ»¯¼ءس뻹ش¼ءµؤخïضتµؤء؟ض®±بتا3:2،£
£¨5£©سةسعCO32£،¢SO42£ء½ضضثل¸ùزُہë×س¶¼ؤـ؛حBa2£«½ل؛دةْ³ةج¼ثل±µ؛حءٍثل±µ°×ة«³ءµي£¬¶ّج¼ثل±µؤـبـ½âشعثلضذ£¬¾ف´ث؟ةزش¼ىرéء½ضضہë×س،£سضزٍخھض»شتذيب،سأز»´خرùئ·£¬ثùزشصب·µؤ²ظ×÷·½·¨تاب،ةظء؟بـز؛aسعتش¹ـضذ£¬µخ¼سBaCl2»ٍBa(NO3)2بـز؛£¬¹آث£¬دٍثùµأ³ءµيضذ£¬µخ¼س¹ء؟µؤرخثل»ٍدُثل£¬³ءµي²؟·ضبـ½â£¬ثµأ÷سذCO32£،¢SO42£،£
؟¼µم£؛؟¼²é¹¤زصء÷³ججâµؤإذ¶د،¢°±ئّضئ±¸؛ح¼ىرé،¢رُ»¯»¹ش·´س¦µؤإذ¶د؛حس¦سأزش¼°ہë×سµؤ¼ىرéµب


 أûذ£ء·؟¼¾يئعؤ©³ه´ج¾يدµءذ´ً°¸
أûذ£ء·؟¼¾يئعؤ©³ه´ج¾يدµءذ´ً°¸
| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛µ¥ر،جâ
2012ؤ궬جى£¬خز¹ْ³اتذ؟صئّخغب¾×´؟ِتـµ½بثأاµؤا؟ءز¹ط×¢،£شع؟صئّضتء؟±¨¸وضذ£¬SO2µؤض¸تتا؛âء؟؟صئّضتء؟؛أ»µµؤضطزھض¸±ê،£خھءث²â¶¨؟صئّضذµؤSO2؛¬ء؟£¬سذبخ»ح¬ر§·ض±ً²ةسأءثزشدآبضض²â¶¨·½·¨،£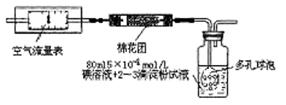
I£®رُ»¯»¹ش·¨£؛¼×ح¬ر§¸ù¾ف»¯ر§·´س¦شہيSO2+I2+2H2O=H2SO4+2HIةè¼ئءثبçدآح¼ثùت¾µؤ×°ضأ½ّذذتµرé£؛
£¨1£©¸أتµرéذè80mlإ¨¶بخھ5،ء10-4mol/Lµؤµâ
بـز؛£¬¼×ح¬ر§س¦ر،شٌ mlµؤبفء؟ئ؟½ّذذإنضئ،£
£¨2£©¹م؟عئ؟ضذت¹سأ¶à؟×اٍإفµؤؤ؟µؤتا ،£
£¨3£©شع¼×ح¬ر§ثùإنµâبـز؛إ¨¶ب×¼ب·£¬²¢ازء؟ب،ز©ئ·¼° تµرé¹³جضذ¸÷ضض¶ءت¾ùخق´يخَµؤاé؟ِدآ£¬ہûسأةدتِ×°ضأثù²â¶¨µؤSO2؛¬ء؟بشب»±بتµ¼ت؛¬ء؟µح£¬اë·ضخِئنضذ؟ةؤـµؤشزٍ£¨ضءةظذ´ء½ضضشزٍ£©
II£®ضطء؟·¨£؛ززح¬ر§ؤâسأتµرéتز³£سأزائ÷×é³ة¼ٍز××°ضأ²â¶¨؟صئّضذµؤSO2؛¬ء؟،£تµرé²ظ×÷¹دآ£؛
°´بçدآتµرé×°ضأح¼°²×°؛أزائ÷£¬شع¹م؟عئ؟ضذت¢·إ×مء؟µؤH2O2ث®بـز؛£¬سأ¹و¸ٌخھ20mlµؤصëح²³éئّ100´خ£¬ت¹؟صئّضذµؤSO2±»H2O2ث®بـز؛³ن·ضخüتص£¨SO2+H2O2=H2SO4£©،£شعخüتص؛َµؤث®بـز؛ضذ¼سبë×مء؟µؤBaCl2بـز؛£¬ةْ³ة°×ة«³ءµي£¨H2SO4+BaCl2=BaSO4،+2HCl£©£¬¾¹آث،¢د´µس،¢¸ةشïµب²½ضè؛َ½ّذذ³ئء؟£¬µأ°×ة«¹ججه0£®18mg،£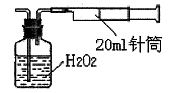
£¨4£©ب،رù´¦؟صئّضذSO2؛¬ء؟خھ mg/L£¨¾«ب·µ½0.001£©،£
£¨5£©دض²éشؤ×تءدضھ£¬³£خآدآBaSO3µؤKspخھ5£®48،ء10-7£¬±¥؛حراءٍثلبـز؛ضذc(SO32-)=6.3،ء10-8mol/L،£سذح¬ر§بدخھزشةدتµرé²»±طسأH2O2خüتصSO2£¬ض±½سسأ0.lmol/L BaCl2بـز؛ہ´خüتصSO2¼´؟ة²ْةْ³ءµي£¬ؤمبدخھصâرù×ِ £¨جî،°صب·،±»ٍ،°²»صب·،±£©£¬اëہûسأزشةدت¾ف¼ٍتِہيسة ،£
III£®زائ÷·¨£؛±ûح¬ر§ض±½ست¹سأز»ضضSO2إ¨¶بضاؤـ¼à²âزا²â¶¨؟صئّضذµؤSO2؛¬ء؟£¬صâضض¼à²âزاتاہûسأµç»¯ر§شہي£¬¸ù¾فµç³ط²ْةْµçء÷µؤا؟¶بہ´×¼ب·²âء؟SO2إ¨¶بµؤ،£¸أµç³ط×ـµؤ»¯ر§·´س¦شہيخھ£؛2SO2+O2+2H2O=2H2SO4،£
£¨6£©اëذ´³ِ¸أµç³ط¸؛¼«µؤµç¼«·´س¦ت½£؛ ،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛µ¥ر،جâ
دآءذتµرé×°ضأتاج½¾؟حث؟سë¹ء؟إ¨ءٍثلµؤ·´س¦£¬دآءذذًتِصب·µؤتا £¨ £©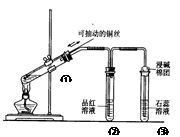
| A£®ةددآزئ¶¯¢ظضذحث؟؟ة؟طضئSO2µؤء؟ |
| B£®¢عضذئ·؛ىبـز؛²»حتة« |
| C£®¢غضذت¯بïبـز؛±نہ¶ة« |
| D£®خھب·بدCuSO4ةْ³ة£¬دٍ¢ظضذ¼سث®£¬¹غ²ىبـز؛رصة« |
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛µ¥ر،جâ
دآءذ»¯ر§تµرé»ٍ²ظ×÷ؤـ¹»´ïµ½ؤ؟µؤµؤتا
| A£®خھ¼ّ±ًKCl،¢AlCl3؛حMgCl2بـز؛£¬·ض±ًدٍبضضبـز؛ضذµخ¼سNaOHبـز؛ضء¹ء؟ |
| B£®سû³ب¥µ°°×ضتبـز؛ضذµؤNaCl¶ّسض²»¸ؤ±نئنذشضت£¬؟ة¼سبëتتء؟BaCl2بـز؛؛َ¹آث |
| C£®²âآبث®µؤpH£¬؟ةسأ²£ء§°ôص؛ب،آبث®µمشعpHتشض½ةد£¬´ئن±نة«؛َ؛ح±ê×¼±بة«؟¨±ب½د |
| D£®خھءثض¤أ÷½¹آ¯ئّضذ؛¬سذاâئّ£¬؟ة½«½¹آ¯ئّح¨¹×ئببµؤرُ»¯ح·غؤ©£¬؟´؛عة«·غؤ©تا·ٌ±ن؛ىة« |
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
بآبزىاèؤٍثل(½ل¹¹¼ٍت½بçح¼)تاز»ضض¼«ا؟µؤرُ»¯¼ء؛حآب»¯¼ء،£
£¨1£©ہûسأبآبزىاèؤٍثلث®½â²ْخïضذµؤرُ»¯ذشخïضتX؟ةدû¶¾أً¾ْ£¬Xµؤ·ض×ست½خھ ،£
£¨2£©،°سذذ§آب،±؛¬ء؟ض¸´سKIضذرُ»¯³ِدàح¬ء؟µؤI2ثùذèCl2µؤضتء؟سëض¸¶¨»¯؛دخïµؤضتء؟ض®±ب£¬³£زش°ظ·ضت±يت¾،£خھ²â¶¨بآبزىاèؤٍثلµؤ،°سذذ§آب،±؛¬ء؟£¬دض³ئب،ؤ³بآبزىاèؤٍثلرùئ·0.5680 g£¬¼سث®،¢×مء؟KI،¢ءٍثل£¬إنضئ³ة100 mL´²âز؛£»×¼ب·ء؟ب،25.00 mL´²âز؛سعµâء؟ئ؟ضذ£¬سأ0.1500 mol،¤L£1 Na2S2O3±ê×¼بـز؛µخ¶¨ضءبـز؛³تخ¢»ئة«ت±£¬¼سبëµي·غض¸ت¾¼ء£¬¼جذّµخ¶¨ضءضصµم(·¢ةْ·´س¦µؤ·½³جت½خھ£؛2Na2S2O3£«I2=Na2S4O6£«2NaI)£»ضط¸´²â¶¨2´خ£¬ثùµأµؤدà¹طت¾فبçدآ±ي£؛
| µخ¶¨ذٍ؛إ | ´²âز؛جه»/mL | ±ê×¼ز؛µخ¶¨¹ـئًµم¶ءت/mL | ±ê×¼ز؛µخ¶¨¹ـضصµم¶ءت/mL |
| 1 | 25.00 | 0.06 | 24.04 |
| 2 | 25.00 | 0.02 | 24.02 |
| 3 | 25.00 | 0.12 | 24.14 |
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛خت´ًجâ
¹رُ»¯أ¾(MgO2)ز×بـسعد،ثل£¬بـسعثل؛َ²ْةْ¹رُ»¯ا⣬شعز½ر§ةد؟ة×÷خھ½âثل¼ءµب،£¹رُ»¯أ¾²ْئ·ضذ³£»ل»ىسذةظء؟MgO£¬تµرéتز؟ةح¨¹¶àضض·½°¸²â¶¨رùئ·ضذ¹رُ»¯أ¾µؤ؛¬ء؟،£ 
£¨1£©ؤ³رذ¾؟ذ،×éؤâسأسزح¼×°ضأ²â¶¨ز»¶¨ضتء؟µؤرùئ·ضذ¹رُ»¯أ¾µؤ؛¬ء؟،£
¢ظتµرéا°ذè½ّذذµؤ²ظ×÷تا £®د،رخثلضذ¼سبëةظء؟FeCl3بـز؛µؤ×÷سأتا ،£
¢عسأ؛مر¹·ضز؛آ©¶·µؤسإµمسذ£؛ت¹·ضز؛آ©¶·ضذµؤبـز؛ث³ہûµخدآ£» ،£
¢غتµرéضصءثت±£¬´»ض¸´ضءتزخآ£¬دب £¬شظئ½تس؟ج¶بدك¶ءت،£
£¨2£©تµرéتز»¹؟ةح¨¹دآءذء½ضض·½°¸²â¶¨رùئ·ضذ¹رُ»¯أ¾µؤ؛¬ء؟£؛
·½°¸I£؛ب،a gرùئ·£¬¼سبë×مء؟د،رخثل£¬³ن·ض·´س¦؛َشظ¼سبë NaOHبـز؛ضءMg2£«³ءµيحêب«£¬¹آث،¢د´µس؛َ£¬½«آثشü³ن·ض×ئةص£¬×îضصµأµ½b g¹ججه،£
·½°¸¢ٍ£؛³ئب،0.1 gرùئ·ضأسعµâء؟ئ؟ضذ£¬¼سبë15 mL0.6 mol/LKIبـز؛؛ح×مء؟رخثل£¬ز،شب؛َشع°µ´¦¾²ضأ5 min£¬ب»؛َسأ0.1 mol/L Na2S2O3بـز؛µخ¶¨£¬µخ¶¨µ½ضصµمت±¹²دû؛ؤVmL Na2S2O3بـز؛،£(زرضھ£؛I2+2Na2S2O3= Na2S4O6+2NaI)
¢ظزرضھ³£خآدآKsp[Mg(OH)2]=l،ء10£11،£خھت¹·½°¸IضذMg2+حêب«³ءµي[¼´بـز؛ضذc(Mg2+)،ـl ،ء10£5mol/L]£¬بـز؛µؤpHضءةظس¦µ÷ضء ،£·½°¸Iضذ¹رُ»¯أ¾µؤضتء؟·ضتخھ (سأ؛¬a،¢bµؤ±ي´ïت½±يت¾)،£
¢ع·½°¸¢ٍضذµخ¶¨ا°ذè¼سبëةظء؟ ×÷ض¸ت¾¼ء£»رùئ·ضذ¹رُ»¯أ¾µؤضتء؟·ضتخھ £¨سأ؛¬Vµؤ±ي´ïت½±يت¾)،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛تµرéجâ
£¨20·ض£©¹¤زµةد³£ہûسأ؛¬ءٍ·دث®ةْ²ْNa2S2O3?5H2O£¬تµرéتز؟ةسأبçدآ×°ضأ£¨آشب¥²؟·ض¼س³ضزائ÷£©ؤ£ؤâةْ³ة¹³ج،£
ةصئ؟Cضذ·¢ةْ·´س¦بçدآ£؛
Na2S£¨aq£©+H2O£¨l£©+SO2£¨g£©=Na2SO3£¨aq£©+H2S£¨aq£© £¨I£©
2H2S£¨aq£©+SO2£¨g£©=3S£¨s£©+2H2O£¨l£© £¨II£©
S£¨s£©+Na2SO3£¨aq£© Na2S2O3£¨aq£© £¨III£©
Na2S2O3£¨aq£© £¨III£©
£¨1£©زائ÷×é×°حê³ة؛َ£¬¹ط±صء½¶ث»îبû£¬دٍ×°ضأBضذµؤ³¤¾±آ©¶·ؤع×¢بëز؛جهضءذخ³ةز»¶خز؛×¢£¬بô £¬شٍصû¸ِ×°ضأئّأـذشء¼؛أ،£×°ضأDµؤ×÷سأتا
،£×°ضأEضذخھ بـز؛،£
£¨2£©خھجل¸ك²ْئ·´؟¶ب£¬س¦ت¹ةصئ؟CضذNa2S؛حNa2SO3ا،؛أحêب«·´س¦£¬شٍةصئ؟CضذNa2S؛حNa2SO3خïضتµؤء؟ض®±بخھ ،£
£¨3£©×°ضأBµؤ×÷سأض®ز»تا¹غ²ىSO2µؤةْ³ةثظآت£¬ئنضذµؤز؛جه×î؛أر،شٌ ،£
a£®صôءَث® b£®±¥؛حNa2SO3بـز؛
c£®±¥؛حNaHSO3بـز؛ d£®±¥؛حNaHCO3بـز؛
تµرéضذ£¬خھت¹SO2»؛آ½ّبëةصئ؟C£¬²ةسأµؤ²ظ×÷تا ،£زرضھ·´س¦£¨III£©دà¶ش½دآ£¬شٍةصئ؟Cضذ·´س¦´ïµ½ضصµمµؤدضدَتا ،£·´س¦؛َئع؟ةسأ¾ئ¾«µئتتµ±¼سببةصئ؟A£¬تµرéتزسأ¾ئ¾«µئ¼سببت±±طذëت¹سأت¯أقحّµؤزائ÷؛¬سذ ،£
a£®ةص± b£®صô·¢أَ c£®تش¹ـ d£®×¶ذخئ؟
£¨4£©·´س¦ضصض¹؛َ£¬ةصئ؟Cضذµؤبـز؛¾صô·¢إ¨ثُ¼´؟ةخِ³ِNa2S2O3?5H2O£¬ئنضذ؟ةؤـ؛¬سذNa2SO3،¢Na2SO4µبشسضت،£ہûسأثù¸ّتش¼ءةè¼ئتµر飬¼ى²â²ْئ·ضذتا·ٌ´وشعNa2SO4£¬¼ٍزھثµأ÷تµرé²ظ×÷£¬دضدَ؛ح½لآغ£؛
،£
زرضھNa2S2O3?5H2Oسِثلز×·ض½â£؛S2O32?+2H+=S،+SO2،ü+H2O
¹©ر،شٌµؤتش¼ء£؛د،رخثل،¢د،ءٍثل،¢د،دُثل،¢BaCl2بـز؛،¢AgNO3بـز؛
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛تµرéجâ
¼îت½ج¼ثلرخA؟ةسأ×÷خ¸ز©£¬ئن×é³ة؟ة±يت¾خھAl2Mg6(OH)x(CO3)y،¤zH2O،£ؤ³ذ£»¯ر§ذثب¤ذ،×éسû²â¶¨ئن»¯ر§ت½£¬تµرéةè¼ئبçدآ£؛
تµرéI£؛³ئب،ز»¶¨ضتء؟µؤA£¬¼سبب·ض½âضء؛مضط،£
تµرé¢ٍ£؛³ئب،ز»¶¨ضتء؟µؤAسë×مء؟µؤثل·´س¦£¬²âء؟ةْ³ةCO2ئّجهµؤضتء؟،£
؟ة¹©ر،شٌµؤزائ÷؛حز©ئ·بçح¼ثùت¾£؛£¨ثلبـز؛دقر،6mol/LHCl»ٍ6mol/LH2SO4£¬ئنثüتش¼ءبخر،،££©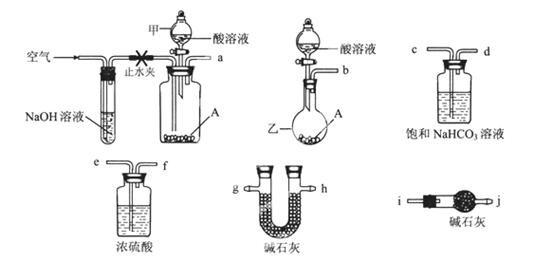
»ط´ًدآءذختجâ£؛
(1)زائ÷ززµؤأû³ئخھ________،£
(2)اëر،شٌ±طزھµؤ×°ضأحê³ةتµرéII,صب·µؤء¬½سث³ذٍخھ________ (°´ئّء÷·½دٍ£¬سأ½س؟ع×ضؤ¸±يت¾£©£»ر،سأµؤثلبـز؛تا________،£
(3)سذبثجل³ِ²»²ةسأتµرéI£¬؟ةشعتµرéII½لتّ؛َ£¬شعAحêب«·´س¦؛َثùµأبـز؛ضذµخ¼س×مء؟µؤ°±ث®£¬سأخق»زآثض½¹آث£¬سأصôءَث®د´µس·´س¦بفئ÷2?3´خ£¬½«د´µسز؛¹آث£¬د´µس³ءµي2?3´خ£¬½«¸½×إ³ءµيµؤآثض½·إµ½غلغِضذ¼سبب·ض½âضء؛مضط،£إذ¶د³ءµيزرد´µس¸ة¾»µؤ·½·¨تا_________________,تµ¼تةدخ´²ةسأ¸أ·½°¸µؤشزٍتا²»·û؛دتµرéةè¼ئµؤ________ششٍ£¨جî×ضؤ¸±à؛إ£©،£
| A£®؟ئر§ذش | B£®°²ب«ذش | C£®؟ةذذذش | D£®¼ٍش¼ذش |
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛µ¥ر،جâ
دآءذ¸÷تµرé×°ضأح¼µؤذًتِضذ£¬صب·µؤتا£¨،،،،£©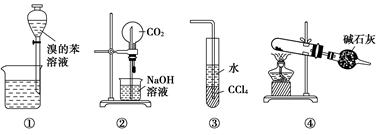
| A£®×°ضأ¢ظخھ·إ³ِفحب،نهث®؛َµؤ±½²م |
| B£®×°ضأ¢عخھإçبھتµرé |
| C£®×°ضأ¢غ؟ةسأہ´خüتصHClئّجه |
| D£®زشNH4Clخھشءد£¬×°ضأ¢ـ؟ةسأسعضئ±¸ةظء؟NH3 |
²é؟´´ً°¸؛ح½âخِ>>
°ظ¶بضآذإ - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com