
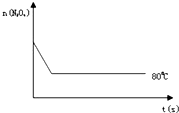
 ��֪N2O4����ɫ��?2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
��֪N2O4����ɫ��?2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�| ʱ�䣨S�� | 0 | 20 | 40 | 60 | 80 | 100 |
| n�� N2O4 ����mol�� | 0.80 | a | 0.40 | c | d | e |
| n��NO2����mol�� | 0.00 | 0.48 | b | 1.04 | 1.20 | 1.20 |
| ||
| ��t |
| ||
| 40s-20s |
| 1 |
| 2 |
| 0.6mol |
| 0.8mol |
| 0.2mol |
| 4L |
| 1.2mol |
| 4L |
| 0��32 |
| 0.05 |
 ��
��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
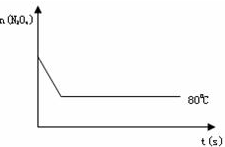
 2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�| ʱ�䣨S�� | 0 | 20 | 40 | 60 | 80 | 100 |
| n�� N2O4 ����mol�� | 0.80 | a | 0.40 | c | d | e |
| n��NO2����mol�� | 0.00 | 0.48 | b | 1.04 | 1.20 | 1.20 |
| c2(NO2) |
| c(N2O4) |
| c2(NO2) |
| c(N2O4) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡȪ��һ��2011�������ѧ����ĩ���Ի�ѧ���� ���ͣ�022
| |||||||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������ض�ģ ���ͣ��ʴ���

| ʱ�䣨S�� | 0 | 20 | 40 | 60 | 80 | 100 |
| n�� N2O4 ����mol�� | 0.80 | a | 0.40 | c | d | e |
| n��NO2����mol�� | 0.00 | 0.48 | b | 1.04 | 1.20 | 1.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ϻ��г����ظ߿���ѧ��ģ�Ծ���4�·ݣ��������棩 ���ͣ������
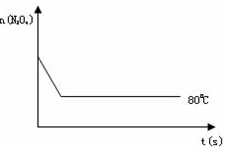
 2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�| ʱ�䣨S�� | 20 | 40 | 60 | 80 | 100 | |
| n�� N2O4 ����mol�� | 0.80 | a | 0.40 | c | d | e |
| n��NO2����mol�� | 0.00 | 0.48 | b | 1.04 | 1.20 | 1.20 |
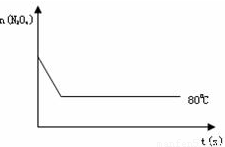
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com