
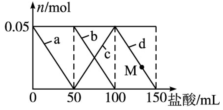
 Ļņ100mLŗ¬Na2CO3”¢NaAlO2µÄ»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė150mL 1mol•L-1HClČÜŅŗ£¬²āµĆČÜŅŗÖŠµÄij¼øÖÖĄė×ÓĪļÖŹµÄĮæµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
Ļņ100mLŗ¬Na2CO3”¢NaAlO2µÄ»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė150mL 1mol•L-1HClČÜŅŗ£¬²āµĆČÜŅŗÖŠµÄij¼øÖÖĄė×ÓĪļÖŹµÄĮæµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | aĒśĻß±ķŹ¾µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAlO2-+H++H2OØTAl£ØOH£©3”ż | |
| B£® | bĒśĻß±ķŹ¾Ģ¼ĖįÄĘŗĶŃĪĖį·“Ó¦£¬dĒśĻß±ķŹ¾ĒāŃõ»ÆĀĮµÄČܽā | |
| C£® | MµćŹ±£¬ČÜŅŗÖŠ³ĮµķµÄÖŹĮæŠ”ÓŚ3.9 g | |
| D£® | Ō»ģŗĻČÜŅŗÖŠµÄNa2CO3ČÜŅŗµÄÅضČĪŖ1 mol•L-1 |
·ÖĪö ĻņNa2CO3”¢NaAlO2µÄ»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė1mol•L-1µÄŃĪĖį£¬ĻČ·¢Éś·“Ó¦AlO2-+H++H2OØTAl£ØOH£©3”ż£¬aĻß±ķŹ¾AlO2-¼õÉŁ£¬µŚ¶ž½×¶Ī£»AlO2-·“Ó¦Ķź±Ļ£¬·¢Éś·“Ó¦CO32-+H+ØTHCO3-£¬bĻß±ķŹ¾CO32-¼õÉŁ£»cĻß±ķŹ¾HCO3-µÄŌö¼Ó£¬µŚČż½×¶Ī£¬CO32-·“Ó¦Ķź±Ļ£¬·¢Éś·“Ó¦HCO3-+H+ØTCO2”ü+H2O£¬dĻß±ķŹ¾HCO3-¼õÉŁ£¬“Ė½×¶ĪAl£ØOH£©3²»²ĪÓė·“Ó¦£¬¾Ż“Ė½įŗĻŃ”Ļī½ųŠŠ½ā“š£®
½ā“š ½ā£ŗNa2CO3”¢NaAlO2µÄ»ģŗĻČÜŅŗÖŠ¼ÓČėHClČÜŅŗ£¬ĻČ·¢Éś·“Ó¦AlO2-+H++H2OØTAl£ØOH£©3”ż£¬aĻß±ķŹ¾AlO2-¼õÉŁ£¬µŚ¶ž½×¶Ī£¬AlO2-·“Ó¦Ķź±Ļ£¬·¢Éś·“Ó¦CO32-+H+ØTHCO3-£¬bĻß±ķŹ¾CO32-¼õÉŁ£¬cĻß±ķŹ¾HCO3-µÄŌö¼Ó£¬µŚČż½×¶Ī£¬CO32-·“Ó¦Ķź±Ļ£¬·¢Éś·“Ó¦HCO3-+H+ØTCO2”ü+H2O£¬dĻß±ķŹ¾HCO3-¼õÉŁ£¬“Ė½×¶ĪAl£ØOH£©3²»²ĪÓė·“Ó¦£¬
A£®Na2CO3£¬NaAlO2µÄ»ģŗĻČÜŅŗÖŠ¼ÓČėHClČÜŅŗ£¬ĻČ·¢Éś·“Ó¦£ŗAlO2-+H++H2OØTAl£ØOH£©3”ż£¬¹ŹAÕżČ·£»
B£®bĒśĻß±ķŹ¾Ģ¼ĖįÄĘŗĶŃĪĖį·“Ó¦£¬dĒśĻß±ķŹ¾Ģ¼ĖįĒāÄĘŗĶŃĪĖį·“Ó¦£¬¹ŹB“ķĪó£»
C£®ŃĪĖį50mLŹ±NaAlO2ÖŠĀĮŌŖĖŲČ«²æ×Ŗ»ÆĪŖĒāŃõ»ÆĀĮ³Įµķ£¬¼Ó50mLŃĪĖįÖ®ŗóCO32-·“Ó¦£¬ĒāŃõ»ÆĀĮ³Įµķ²»Čܽā£¬ŌņMµć³ĮµķµÄÖŹĮæŗĶŃĪĖį50mLŹ±³ĮµķµÄÖŹĮæĻąĶ¬£¬ÓÉNaAlO2+HCl+H2O=NaCl+Al£ØOH£©3”żÖŖ£¬n£ØAl£ØOH£©3£©=n£ØNaAlO2£©=n£ØHCl£©0.05mol£¬m[Al£ØOH£©3]=0.05mol”Į78g/mol=3.9g£¬¹ŹC“ķĪó£»
D£®øł¾Ż·ÖĪöæÉÖŖ£¬n£ØCO32-£©=n£ØH+£©=0.05L”Į1mol/L=0.05mol£¬Ōņc£ØCO32-£©=$\frac{0.05mol}{0.1L}$=0.5 mol•L-1£¬¹ŹD“ķĪó£»
¹ŹŃ”A£®
µćĘĄ ±¾Ģā漲鷓ӦÓėĶ¼ĻóµÄ¹ŲĻµ£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·Ķ¼ĻóÖŠø÷ĢõĒśĻß±ķŹ¾µÄĪļÖŹŹĒ½ā±¾ĢāµÄ¹Ų¼ü£¬×¢ŅāĢ¼ĖįÄĘŗĶŃĪĖį·“Ó¦ŹĒ·Ö²½½ųŠŠµÄ£¬ĻČÉś³ÉĢ¼ĖįĒāÄĘ£¬Č»ŗóĢ¼ĖįĒāÄĘŌŁŗĶŃĪĖį·“Ӧɜ³ÉĀČ»ÆÄĘŗĶĖ®”¢¶žŃõ»ÆĢ¼£®


 Š”ѧĶ¬²½ČżĮ·ŗĖŠÄĆܾķĻµĮŠ“š°ø
Š”ѧĶ¬²½ČżĮ·ŗĖŠÄĆܾķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÉżøßĪĀ¶ČæÉŹ¹øĆ·“Ó¦µÄÄę·“Ó¦ĖŁĀŹ½µµĶ | |
| B£® | Ź¹ÓĆøߊ§“߻ƼĮÖ»ÄÜĢįøßÕż·“Ó¦ĖŁĀŹ | |
| C£® | ·“Ó¦“ļµ½Ę½ŗāŗó£¬NOµÄ·“Ó¦ĖŁĀŹĪŖĮć | |
| D£® | µ„Ī»Ź±¼äÄŚĻūŗÄCOŗĶCO2µÄĪļÖŹµÄĮæĻąµČŹ±£¬·“Ó¦“ļµ½Ę½ŗā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀŹ±£¬12gŹÆÄ«¾§ĢåÖŠĖłŗ¬ĮłŌŖ»·ŹżÄæĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬18g D2OÖŠĖłŗ¬µē×ÓŹżĪŖ9 NA | |
| C£® | 1mol N2Óė 4mol H2·“Ӧɜ³ÉµÄNH3·Ö×ÓŹżĪŖ2NA | |
| D£® | ±ź×¼×“æöĻĀ£¬2.24L SO3ÖŠĖłŗ¬Ō×ÓŹżĪŖ0.4 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com