
����Ŀ��ij�������ֻ������Ե����̼�������缫���ϣ�������ZnSO4��Һ���л��߾�������̬����ʣ����ؽṹ��ͼ����ʾ��ͼ�����л��߾���ĽṹƬ�Ρ�
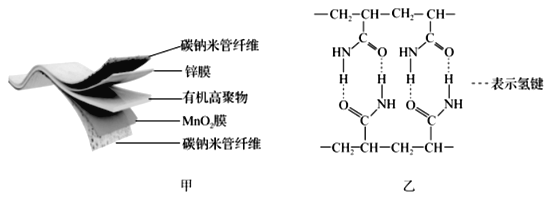
��1��Mn2+�ĺ�������Ų�ʽΪ______________���л��߾�����C���ӻ���ʽΪ_____��
��2����֪CN-��N2��Ϊ�ȵ����壬������±��(CN)2������������������Ŀ֮��Ϊ____��
��3��NO2-�Ŀռ乹��Ϊ__________��
��4��MnO�����Ӿ��壬�侧���ܿ�ͨ����ͼ��Born��Haberѭ������õ���
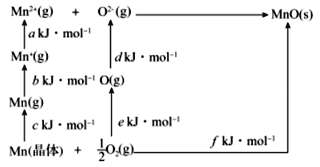
Mn�ĵ�һ��������____��O2�ļ�����____��MnO�ľ�������____��
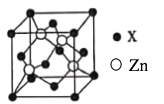
��5��п��ij�ǽ���Ԫ��X�γɵĻ����ᄃ����ͼ��ʾ���谢���ӵ���������ֵΪNA���þ�����Zn�İ뾶Ϊr1 nm�� X�İ뾶Ϊr2 nm��X�����ԭ������ΪM����þ�����ܶ�Ϊ___________g��cm��3(�ú�r1��r2��M��NA�Ĵ���ʽ��ʾ)��
���𰸡�1s22s22p63s23p63d5�� [Ar]3d5 sp2��sp3 3:4 V�� b kJ��mol-1 2e kJ��mol-1 (f-a-b-c-d-e) kJ��mol-1 ![]()
��������
��1��Mn��25��Ԫ�أ����Mn��������Ų�ʽΪ1s22s22p63s3p63d54s2�����Mn2+�ĺ�������Ų�ʽΪ1s22s22p63s3p63d5���л��߾����У�CH2����C�۲���Ӷ���Ϊ4��Ϊsp3�ӻ�����CONH2��C�۲���Ӷ���Ϊ3��Ϊsp2�ӻ����ʴ�Ϊ��1s22s22p63s23p63d5�� [Ar]3d5��sp2��sp3��
��2����֪CN����N2��Ϊ�ȵ����壬�Ƴ���±��(CN)2���ӽṹΪN��C��C��N��������������������Ŀ֮��Ϊ3:4���ʴ�Ϊ��3:4��
��3��NO2���۲���Ӷ���Ϊ![]() ����˿ռ乹��Ϊ��V���Σ��ʴ�Ϊ��V�Ρ�
����˿ռ乹��Ϊ��V���Σ��ʴ�Ϊ��V�Ρ�
��4����һ�����ܶ�������̬��̬������ʧȥһ�����ӱ�Ϊ��̬+1�������������յ����������Mn�ĵ�һ��������b kJ��mol1��������1mol���ʶ��ѳ�ԭ�������յ����������O2�ļ�����2e kJ��mol1������������̬�����γ�1mol�������ͷŵ�������MnO�ľ�������(f-a-b-c-d-e) kJ��mol-1���ʴ�Ϊ��b kJ��mol1��2e kJ��mol1��(f-a-b-c-d-e) kJ��mol-1��
��5��п��ij�ǽ���Ԫ��X�γɵĻ����ᄃ����ͼ��ʾ������X����Ϊ![]() ��Zn����Ϊ4����˻�ѧʽΪZnX���谢���ӵ���������ֵΪNA���þ�����Zn�İ뾶Ϊr1 nm��X�İ뾶Ϊr2 nm�����ݽṹ������Խ��ߵ��ķ�֮һΪ��ԭ�Ӱ뾶�ͣ����ⳤΪa������ r1+ r2 =
��Zn����Ϊ4����˻�ѧʽΪZnX���谢���ӵ���������ֵΪNA���þ�����Zn�İ뾶Ϊr1 nm��X�İ뾶Ϊr2 nm�����ݽṹ������Խ��ߵ��ķ�֮һΪ��ԭ�Ӱ뾶�ͣ����ⳤΪa������ r1+ r2 = ![]() ��
��![]() ��X�����ԭ������ΪM����þ�����ܶ�Ϊ
��X�����ԭ������ΪM����þ�����ܶ�Ϊ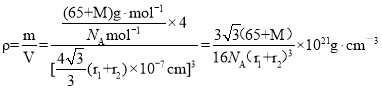 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������A����ԭAʱ�γɴ�B������Aʱ�γ���C��B��C��Ӧ�����ɸ߷��ӻ�����![]() �����������������( )
�����������������( )
A.A����ȩ�࣬����Է�������Ϊ58��1 mol A��������������Һ��Ӧ������4 mol Ag
B.B��һ�������¿�ͨ�����۷�Ӧ�õ��µĸ߾���
C.�߷��ӻ�����![]() �������B��C�����ʵ����Ļ����������ͬ
�������B��C�����ʵ����Ļ����������ͬ
D.B��C���ɸø߾���ķ�ӦΪ���۷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ����ʹ̼���������ǣ� ��
A.CH3CH2CH2CH2Br��NaCN����
B.CH3CH2CH2CH2Br��NaOH���Ҵ���Һ����
C.CH3CH2CH2CH2Br��NaOH��ˮ��Һ����
D.CH3CH2CH2CH2Br(g)��Br2(g)����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������A����ԭAʱ�γɴ�B������Aʱ�γ���C��B��C��Ӧ�����ɸ߷��ӻ�����![]() �����������������( )
�����������������( )
A.A����ȩ�࣬����Է�������Ϊ58��1 mol A��������������Һ��Ӧ������4 mol Ag
B.B��һ�������¿�ͨ�����۷�Ӧ�õ��µĸ߾���
C.�߷��ӻ�����![]() �������B��C�����ʵ����Ļ����������ͬ
�������B��C�����ʵ����Ļ����������ͬ
D.B��C���ɸø߾���ķ�ӦΪ���۷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
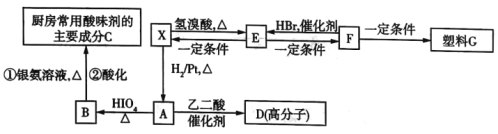
����Ŀ����ij�л���XΪԭ�Ͽɺϳ�����G��X����Է�������С��100��![]() �л���X��ȫȼ�����ɵ����ʵ�����
�л���X��ȫȼ�����ɵ����ʵ�����![]() ��
��![]() ��ͬʱ���ı�״���µ�
��ͬʱ���ı�״���µ�![]() ����X�����к����ʻ����ǻ���X�ܷ�����ͼ��ʾ��ת����
����X�����к����ʻ����ǻ���X�ܷ�����ͼ��ʾ��ת����
��֪�� ![]() ��
��![]() ����������������
����������������![]() ��
��![]() ��
��
�ش��������⣺
(1)X�Ľṹ��ʽΪ_________________��
(2)![]() �ķ�Ӧ������__________��
�ķ�Ӧ������__________��
(3)д��![]() �Ļ�ѧ����ʽ��___________________���÷�Ӧ�ķ�Ӧ����Ϊ__________________��
�Ļ�ѧ����ʽ��___________________���÷�Ӧ�ķ�Ӧ����Ϊ__________________��
(4)![]() �Ļ�ѧ����ʽΪ________________________________���÷�Ӧ�ķ�Ӧ����Ϊ_________��
�Ļ�ѧ����ʽΪ________________________________���÷�Ӧ�ķ�Ӧ����Ϊ_________��
(5)Y��X��ͬ���칹�壬![]() ��������
��������![]() ��Ӧ������
��Ӧ������![]() ����Y����ʹ���
����Y����ʹ���![]() ��Һ��ɫ��Y�����еĹ������������ڵ�̼ԭ���ϡ�Y�ĺ˴Ź�������ͼ����3���壬�����֮��Ϊ2��1��1��PBS��һ���������ϣ���ṹ��ʽΪ
��Һ��ɫ��Y�����еĹ������������ڵ�̼ԭ���ϡ�Y�ĺ˴Ź�������ͼ����3���壬�����֮��Ϊ2��1��1��PBS��һ���������ϣ���ṹ��ʽΪ![]() ������ƺ���������YΪԭ��(���Լ���ѡ)�ϳ�PBS��________________________(�úϳ�·������ͼ��ʾ����ע����Ӧ����)��
������ƺ���������YΪԭ��(���Լ���ѡ)�ϳ�PBS��________________________(�úϳ�·������ͼ��ʾ����ע����Ӧ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ�Ӿ�������ԭ��ֱ��ͨ�����ۼ��γɵĿռ���״�ṹ�ľ��壬�ֳƹ��۾��壬������и��۷е㡢Ӳ�ȴ���ĥ���������Զ����й㷺����;��
(1)����������õİ뵼����ϣ����㷺������Ϣ��������Դ��ѧ���������������ʯ�ṹ���Ƶľ���(�侧����ͼ����ʾ)���辧���1�������к�_____��Siԭ�ӣ��ھ����Ŀռ���״�ṹ����С��Ϊ_____Ԫ����ÿ��С����������__________��Siԭ�ӣ���1molSiԭ�ӵľ������Si-Si������ĿΪ_____��
(2)���ɰ(SiC)Ҳ����ʯ�������Ƶľ���ṹ(��ͼ����ʾ)���ڽ��ɰ�Ŀռ���״�ṹ�У�̼ԭ�ӡ���ԭ�ӽ����Թ��۵������ϡ��Իش��������⣺
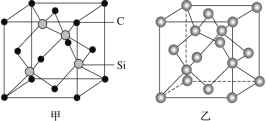
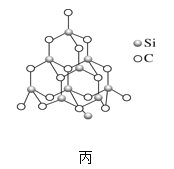
�ٽ��ɰ�����ʯ���������۵��ɵ͵��ߵ�˳����_____(���û�ѧʽ��ʾ)��
���ڽ��ɰ�Ľṹ�У�һ��̼ԭ����Χ�����_____���ԭ�ӣ��������_____��
�۽��ɰ�Ľṹ�к���C��Siԭ���Թ��ۼ�����γɵĻ�������һ����С�Ļ��϶�������_��C-Si��
�ܽ��ɰ�ľ����ṹ��ͼ����ʾ����SiC�У�ÿ��Cԭ����Χ����Ⱦ��Cԭ����ĿΪ_____�������ɰ���ܶ�Ϊ��g��cm-3�������ӵ�����ΪNA�����������������̼��ԭ��֮��ľ���Ϊ_________pm(�ô���ʽ��ʾ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���л���A��![]() ����
����![]() ��Һ��Ӧ�Գ���ɫ�ұ�����������ȡ������ͬ���칹����___�֣��Էֱ�д����ṹ��ʽ��___��
��Һ��Ӧ�Գ���ɫ�ұ�����������ȡ������ͬ���칹����___�֣��Էֱ�д����ṹ��ʽ��___��
��2��M�Ļ�ѧʽΪC4H9Cl����֪A�ĺ˴Ź������ױ�����ֻ��һ����ԭ�ӣ���M�Ļ�ѧ����Ϊ___��
��3��д��ͬʱ�������������ķ���ʽΪC6H10O4�����нṹ��ʽ��___��
��ֻ��һ�ֹ����Ţ���״�ṹ���ޡ�O��O���ۺ˴Ź�������ֻ��2���
��4��ij������B��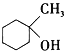 ��ͬ���칹�壬�ҷ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�д���û�����Ľṹ��ʽ��___����дһ�֣���
��ͬ���칹�壬�ҷ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�д���û�����Ľṹ��ʽ��___����дһ�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.6mol KCl��0.4mol Cu(NO3)2��0.2molAgNO3һ������ˮ�����100mL�����Һ���ö��Ե缫���һ��ʱ�������һ������19.2g Cu����ʱ����һ���ϲ���������������״���£�Ϊ
A.3.96LB.4.48LC.5.6LD.6.72L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��pC����pH����ָ��ϡ��Һ�У��������ʵ���Ũ�ȵij��ö�����ֵ����ij��Һ���ʵ�Ũ��Ϊ1��10��3mol��L��1�������Һ�����ʵ�pC=��lg(1��10��3)=3����ͼΪH2CO3�ڼ���ǿ���ǿ����Һ��ƽ��ʱ��Һ�����ֳɷֵ�pC��pHͼ������˵������ȷ����

A. H2CO3��HCO3-��CO32-������ͬһ��Һ�д�������
B. H2CO3����ƽ�ⳣ��Ka1��10��6
C. pH=7ʱ����Һ�д��ڹ�ϵc(HCO![]() )��c(H2CO3)��c(CO
)��c(H2CO3)��c(CO![]() )
)
D. pH=9ʱ����Һ�д��ڹ�ϵc(H��)��c(H2CO3)=c(OH��)��c(CO![]() )
)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com