
 PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ�����������
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ�����������| �۵�/�� | �е�/���� | �ܶ�/g?cm-3 | ���� | |
| ���� | 44.1 | 280.5 | 1.82 | 2P��������+3Cl2 2PCl3��2P+5Cl2�������� 2PCl3��2P+5Cl2�������� 2PCl5 2PCl5 |
| PCl3 | -112 | 75.5 | 1.574 | ��ˮ����H3PO3��HCl����O2����POCl3 |
| POCl3 | 2 | 105 3 | 1.675 | ��ˮ����H3PO4��HC1��������PCl3 |
 ��c2��V2��10-3mol��������H3PO3��Ӧ��I2Ϊc1mol?L-1��V1��10-3L-
��c2��V2��10-3mol��������H3PO3��Ӧ��I2Ϊc1mol?L-1��V1��10-3L- ��c2��V2��10-3mol=��c1V1-
��c2��V2��10-3mol=��c1V1- c2V2����10-3mol��
c2V2����10-3mol�� c2V2����10-3mol��
c2V2����10-3mol��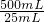 =��c1V1-
=��c1V1- c2V2����20��10-3mol��
c2V2����20��10-3mol�� c2V2����20��10-3mol��137.5g/mol=2.75��c1V1-
c2V2����20��10-3mol��137.5g/mol=2.75��c1V1- c2V2��g��
c2V2��g��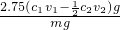 ��100%=
��100%=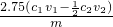 ��100%��
��100%��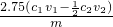 ��100%��
��100%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�| �۵�/�� | �е�/���� | �ܶ�/g?cm-3 | ���� | |||||||||
| ���� | 44.1 | 280.5 | 1.82 | 2P��������+3Cl2
| ||||||||
| PCl3 | -112 | 75.5 | 1.574 | ��ˮ����H3PO3��HCl����O2����POCl3 | ||||||||
| POCl3 | 2 | 105 3 | 1.675 | ��ˮ����H3PO4��HCl��������PCl3 |
2.75(c1v1-
| ||
| m |
2.75(c1v1-
| ||
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �۵�/�� | �е�/�� | �ܶ�/g?mL | ���� | |||||||||
| ���� | 44.1 | 280.5 | 1.82 | 2P��������+3Cl2
2P+5Cl2��������
| ||||||||
| PCl3 | -112 | 75.5 | 1.574 | ��ˮ����H3PO3��HCl����O2����POCl3 | ||||||||
| POCl3 | 2 | 105.3 | 1.675 | ��ˮ����H3PO4��HCl�������� PCl3 |
| ||
| ||
(c1V1-
| ||
| m |
(c1V1-
| ||
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
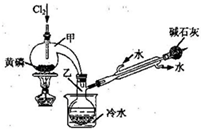
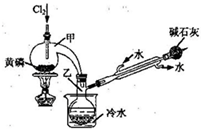
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���
��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
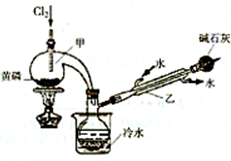
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���
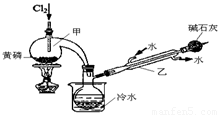
��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
|
|
�۵�/�� |
�е�/�� |
�ܶ�/g��mL��1 |
���� |
|
���� |
44��1 |
280��5 |
1��82 |
2P(����)+3Cl2 |
|
PCl3 |
��112 |
75��5 |
1��574 |
��ˮ����H3PO3��HCl����O2����POCl3 |
|
POCl3 |
2 |
105��3 |
1��675 |
��ˮ����H3PO4��HCl��������PCl3 |
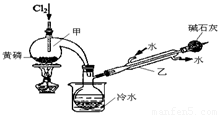
��ͼ��ʵ�����Ʊ�PCl3��װ�ã�����������ʡ�ԣ���

��1�������ͨ������Cl2֮ǰ������ͨ��һ��ʱ��CO2��Ŀ���� ��
��2��ʵ������У�Ӧ���� ���Լ���PCl5�����ɡ�
��3���ֲ�ƷƷ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ�� ����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
��4����֪��a��H3PO3+H2O+I2
 H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
H3PO4+2HI��b��(NH4)3BO3��Һ����HI��H3PO4��Ӧ����H3BO3�����ᣩ��c��Na2S2O3��Һ�ɶ����ⶨ�⣺I2+2Na2S2O3��Na2S4O6+2NaI
�ٲⶨ��Ʒ��PCl3����������ʵ�����£��벹����Ӧ��ʵ�鲽�裺
����1��Ѹ����ȡm g��Ʒ��ˮ����ȫ����500mL����ƿ�ж��ݡ�
����2��������ƿ����ȡ25��00mL��Һ������ƿ�С�
����3��ȷ����c1 mol/L����ҺV1 mL������������ ��
����4���ñ�Na2S2O3��Һ�صι����ĵ⣬�����յ�ʱ����3 mL������Һ�����������յ㣨��ɫ��Һ��ɫ���������յ�ʱ����c2 mol/L Na2S2O3��ҺV2 mL��
�ڸ����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com