
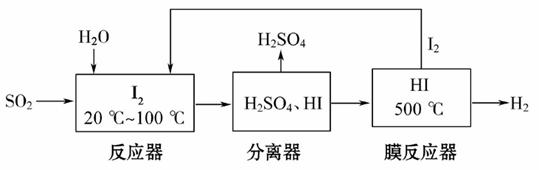
����������ʵ�����ɳ�����չ����Ҫ��������(FeS2)ȼ�ղ�����SO2ͨ�����е�ѭ�����չ��̼�����H2SO4��������H2��
��ش��������⣺
(1)��֪1 g FeS2��ȫȼ�շų�7.1 kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ
________________________________________��
(2)��ѭ�����չ��̵��ܷ� Ӧ����ʽΪ__________________________________��
Ӧ����ʽΪ__________________________________��
(3)�û�ѧƽ���ƶ���ԭ����������HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H2��Ŀ����__________________________________��
(4)������H2���ϡ������Ͻ���Ϊ��ظ�������(��MH��ʾ)��NiO(OH)��Ϊ����������ϣ�KOH��Һ��Ϊ�������Һ�����Ƶø��������������������ء���س�ŵ�ʱ���ܷ�ӦΪ��
NiO(OH)+MH Ni(OH)2+M
Ni(OH)2+M
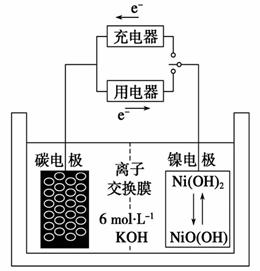
�ٵ�طŵ�ʱ�������ĵ缫��ӦʽΪ_________________________________��
�ڳ�����ʱ��Ni(OH)2ȫ��ת��ΪNiO(OH)����������罫��һ���缫����O2��O2��ɢ����һ���缫�����缫��Ӧ�����ģ��Ӷ�������������������ر�ը����ʱ�������ĵ缫��ӦʽΪ___________________________________________��
(1)��Ӧ����ʽ��4FeS2��11O2====2Fe2O3��8SO2����������ʵľۼ�״̬���ڷ�Ӧ��4 mol FeS2������Ϊm(FeS2)=4 mol��120 g��mol��1=480 g������Q=480 g��7.1 kJ��g-1=3 408 kJ����Ӧ���Ȼ�ѧ����ʽΪ��
4FeS2(s)��11O2(g)====2Fe2O3(s)��8SO2(g) ��H=��3 408 kJ��mol��1��
(2)�ڷ�Ӧ���з�����Ӧ��SO2��I2��2H2O====2HI��H2SO4����Ĥ��Ӧ�� �еķ�ӦΪ��2HI
�еķ�ӦΪ��2HI I2��H2������������ʽ��ӵã�SO2��2H2O====H2SO4��H2��
I2��H2������������ʽ��ӵã�SO2��2H2O====H2SO4��H2��
(3)��Ĥ�������з�����Ӧ��2HI I2��H2����H2�������������ƽ�������ƶ�������I2��H2�����ɡ�
I2��H2����H2�������������ƽ�������ƶ�������I2��H2�����ɡ�
(4)�ٸ�����Ӧ��MHʧȥ���ӣ����ɵ�H���ڼ�������������H2O���缫��ӦʽΪMH��e����OH��====H2O��M��
��O2��MH������Ӧ������H2O��M���缫��ӦʽΪ4MH��O2��4e��==== 2H2O��4M��
2H2O��4M��
�𰸣�(1)4FeS2(s)��11O2(g)====2Fe2O3(s)��8SO2(g) ��H=��3 408 kJ��mol��1
(2)SO2��2H2O====H2SO4��H2
(3)��ʹƽ�������ƶ��������ڵ������������
(4)��MH��e����OH��====H2O��M
��4MH��O2��4e��====2H2O��4M


 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�������ʽṹ�����ʡ�
���������з�Ӧ�ϳ������谷��CaO��3C CaC2��CO����CaC2��N2
CaC2��CO����CaC2��N2  CN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
CN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
��1��д����Ca��ͬһ������������������ͬ���ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽ�� �� CaCN2��������ΪCN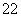 �����ݵȵ���ԭ��������֪CN
�����ݵȵ���ԭ��������֪CN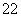 �Ŀռ乹��Ϊ ��
�Ŀռ乹��Ϊ ��
 ��2�����ط�����Cԭ�Ӳ�ȡ �ӻ������ط��ӵĽṹ��ʽ�� ��
��2�����ط�����Cԭ�Ӳ�ȡ �ӻ������ط��ӵĽṹ��ʽ�� ��
��3�������谷( )�׳ơ����������������������谷����������( )
�����������������谷�����֮��ͨ�� ��ϣ������������γɽ�ʯ��
��4��CaO������ͼ��ʾ��CaO������Ca2+����λ��Ϊ ��
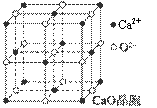

��5��CaO�����NaCl����ľ����ֱܷ�Ϊ��CaO 3401 kJ��mol-1��NaCl 786 kJ��mol-1������CaO��NaCl�����ܴ����Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʢ��ϡH2SO4���ձ��з����õ������ӵ�пƬ��ͭƬ������������ȷ����(����)
A������������SO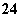 ����Ũ��������
����Ũ��������
B������ͨ��������ͭƬ����пƬ
C��������O2�ݳ�
D��ͭƬ����H2�ݳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�ֻ�������ȼ�ϵ��ԭ����Ƶľƾ�����ǣ������ϵķ�ӦΪCH3CH2OH-4e-+H2O====CH3COOH+4H+�������й�˵����ȷ����( )
A.���ʱ���������Һ�е�H+���ƶ�
B.����0.4 mol����ת�ƣ����ڱ�״��������4.48 L����
C.��ط�Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+O2====CH3COOH+H2O
D�������Ϸ����ķ�Ӧ�ǣ�O2+4e-+2H2O====4OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ�����û�ѧƽ���ƶ�ԭ�����͵���( )
A����ˮ��������ƽ��Br2+H2O HBr+HBrO����������������Һ����Һ��ɫ��dz
HBr+HBrO����������������Һ����Һ��ɫ��dz
B���ϳɰ���ӦΪ��߰��IJ��ʣ�������Ӧ��ȡ�����¶ȵĴ�ʩ
C����ӦCO(g)+NO2(g) CO2(g)+NO(g)(����ӦΪ���ȷ�Ӧ)����ƽ��������¶���ϵ��ɫ����
CO2(g)+NO(g)(����ӦΪ���ȷ�Ӧ)����ƽ��������¶���ϵ��ɫ����
D������2HI(g) H2(g)+I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
H2(g)+I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״���CO(g)+2H2(g) CH3OH(g)������˵����ȷ����( )
CH3OH(g)������˵����ȷ����( )
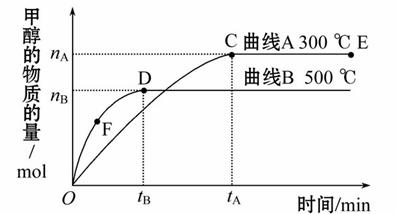
A.E���ƽ�ⳣ������D���ƽ�ⳣ�����Ҹ÷�Ӧ�Ħ�H��0
B.����������������ʵ���E�����D��
C.F�������Ӧ���ʴ����淴Ӧ����
D.v(�״�)��ʾ500 ��ʱ���Ϸ�Ӧ��D�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ����ȷ����( )
A�������ʯ��ˮ��ϡ���ᷴӦ��Ca(OH)2��2H��====Ca2����2H2O
B������ˮ�ķ�Ӧ��Na��2H2O====Na����2OH����H2��
C��ͭƬ������������Һ�У�Cu��Ag��====Cu2����Ag
D������ʯ���ڴ���ķ�Ӧ��CaCO3��2CH3COOH====Ca2����2CH3COO����CO2����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�״������ǿ������õ�һ������Դ����֪��
��2H2(g)��O2(g)===2H2O(l)����H1����571.8 kJ·mol��1
��CH3OH(g)��1/2O2(g)===CO2(g)��2H2(g)����H2����192.9 kJ·mol��1
��CH3OH(l)===CH3OH(g)����H3����37.4 kJ·mol��1
(1)д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ��__________________________________________________________
__________________________________________________________��
(2)H2��ȼ����Ϊ__________________________________________________________��
(3)������Ҳ��һ������ȼ�ϣ�1 mol������������ȫȼ������CO2��Һ̬ˮʱ�ų�1 455 kJ��������1 mol�����Ѻͼ״��Ļ��������ȫȼ������CO2��Һ̬ˮʱ���ų�1 224.9 kJ���������������м״��Ͷ����ѵ����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com