
| A�� | c��PO43-����Na3PO4��Na2HPO4��NaH2PO4��H3PO4 | |
| B�� | c��CO32-������NH4��2CO3��Na2CO3��NaHCO3��NH4HCO3 | |
| C�� | c��NH4+������NH4��2SO4����NH4��2CO3��NH4HSO4��NH4Cl | |
| D�� | c��S2-����Na2S��NaHS��H2S |
���� A����������ߵ������c��H+�������Բ���PO43-�ĵ������������ã�
B��̼������̼�������Ũ�ȴ���̼���������Ũ�ȣ�Ȼ������ε�ˮ��Ӱ���ж�̼�������Ũ�ȴ�С��
C����������狀�̼����е�笠�����Ũ�ȴ�����ʽ����笠�����Ũ�ȣ����������������������笠����ӵĵ��룻
D��������������Ũ����������Ƕ�Ԫ���ᣬ������Ũ����С��
��� �⣺A��Na3PO4�����������Ũ�����Na2HPO4��NaH2PO4��H3PO4�������c��H+�������������ӶԲ���PO43-�ĵ������������ã���Һ�����������Ũ�ȴ�СΪ��Na3PO4��Na2HPO4��NaH2PO4��H3PO4����A��ȷ��
B��Na2CO3�������ǣ�NH4��2CO3����Ϊ����Ҫ����ˮ�⣬NaHCO3��NH4HCO3����HCO3-�����������NH4HCO3��HCO3-��NH4+��ٽ�ˮ�⣬HCO3-Ũ�Ƚ�С��NaHCO3��NH4HCO3����B����
C�������ξ���ȫ���룬��NH4��2SO4 �ͣ�NH4��2CO3�ϴ����ߵ��������ӻᷢ����ٽ���ˮ�⣬ӦΪ��NH4��2SO4����NH4��2CO3��NH4HSO4��NH4Cl��NH4HSO4 ���������H+��NH4+��ˮ�����������ã�ӦΪNH4HSO4��NH4Cl����Һ��笠�����Ũ�ȴ�СΪ����NH4��2SO4����NH4��2CO3��NH4HSO4��NH4Cl����C��ȷ��
D��Na2S���H2S��NaHS��ȣ���ǰ����������������������ӵĵ��룬������Ũ��ӦΪNaHS��H2S����Һ��������Ũ�ȴ�СΪ��Na2S��NaHS��H2S����D��ȷ��
��ѡB��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ������Ӱ��Ϊ���ؼ���ע������������ʵĵ���ƽ�⼰��Ӱ�죬����������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�11.2 L�����к��е���ԭ����Ϊ2NA | |
| B�� | ��״���£�0.3 mol������̼�к�����ԭ����Ϊ0.3 NA | |
| C�� | �����£�2.7 g�������������ᷴӦ��ʧȥ�ĵ�����0.3 NA | |
| D�� | �����£�0.1 mol/L MgCl2��Һ�к�Cl-��Ϊ0.2 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�ŨH2SO4�����������������������棬˵��ŨH2SO4��Al����Fe�������²���Ӧ | |
| B�� | �������м���ŨH2SO4����ַ�������˵��ŨH2SO4������ˮ�� | |
| C�� | ŨH2SO4��ʹ����������ף�˵��ŨH2SO4������ˮ�� | |
| D�� | ϡH2SO4����Fe��Ӧ����H2��˵��ϡH2SO4Ҳ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
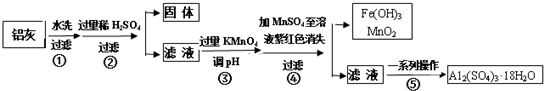
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���ʽṹ�����������ʣ��ش��������⣺
���ʽṹ�����������ʣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | �� | |
| �� | �������� | �����ӵ����� |
| �� | ���������Ħ����� | ������������ |
| �� | �ܼ������ | ���ʵ����ʵ���Ũ�� |
| �� | ��Һ�����ʵ��������� | ��Һ������ |
| �� | �DZ�������ʵ����� | ���ʵ�Ħ������ |
| A�� | �� | B�� | �ۢ� | C�� | �ڢۢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ԭ�ӵĸ�����Ϊ2��3 | B�� | ������ԭ�ӵĸ�����Ϊ1��1 | ||
| C�� | ������Ԫ�ص�������Ϊ5��6 | D�� | ������Ԫ�ص�������Ϊ5��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
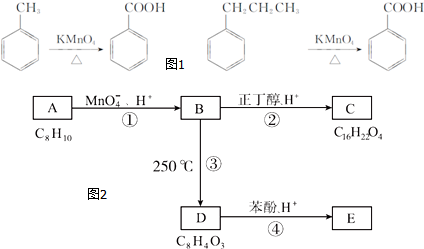
 ��
��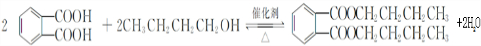 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ��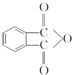 ����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ1��1��
����D���ʵĺ˴Ź�������ͼ�У������2��壬�����֮��Ϊ1��1��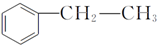 ��д�ṹ��ʽ����
��д�ṹ��ʽ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com