
Ä³Ń§Ļ°Š”×éŌŚŹµŃéŹŅÄ£ÄāŗīŹĻÖĘ¼ī·ØÖʱø“æ¼ī£¬Ę䏵ŃéĮ÷³ĢČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ź³ŃĪČÜŅŗÖŠŗ¬SO42£µČŌÓÖŹ£¬²»ÄÜÓĆÓŚ³żČ„SO42£µÄŹŌ¼ĮŹĒ_________£ØĢīŠņŗÅ£©”£
a£® Ba(OH)2 b£®Ba(NO3)2 c£® BaCl2 d£® Na2CO3
£Ø2£©ŹµŃéÖʱøĢ¼ĖįĒāÄĘÓƵ½ĻĀĮŠ×°ÖĆ£ŗ

¢ŁŹµŃé×°ÖĆÖŠø÷½ÓæŚµÄĮ¬½ÓŹĒd½Ó____£¬e½Ó_____£¬c½Óf”£ŹµŃé¹ż³ĢÖŠĻČĶØČė°±ĘųµÄŌŅņ_____________”£
¢ŚĪö³ö¾§ĢåŗóµÄČÜŅŗÖŠŗ¬ÓŠ“óĮæµÄNH4£«£¬¼ģŃéNH4£«µÄ·½·ØŹĒ__________________”£
¢ŪĘægÄŚµÄČÜŅŗŹĒ___________________________________________”£
£Ø3£©ÓÉĢ¼ĖįĒāÄĘÖʱø“æ¼īµÄ¹ż³ĢÖŠÓƵ½µÄÖ÷ŅŖŅĒĘ÷³ż¾Ę¾«µĘ”¢ÄąČż½Ē”¢Čż½Å¼Ü”¢²£Į§°ōĶā£¬»¹ÓŠ__________”£
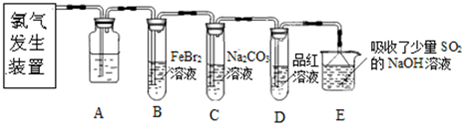
£Ø4£©ÓĆĶ¼µē½ā±„ŗĶNaC1ČÜŅŗµÄ·½·ØĄ“ÖĘČ”NaOH”¢C12ŗĶH2”£

¢Ł·“Ó¦ÖŠÉś³ÉµÄC12“Ó_______£ØĢī”°A”±»ņ”°B”±£©·Å³ö£¬¼ģŃéC12ĖłÓĆŹŌ¼Į£ØÓĆĘ·£©______________________”£
¢Ś¾¹ż³¤Ź±¼äµē½āŗó£¬Ļņµē½āŅŗÖŠµĪ¼Ó·ÓĢŖŹŌŅŗ£¬·¢ĻÖČÜŅŗ²¢Ī“±äŗģÉ«£¬ŹŌ·ÖĪöæÉÄܵÄŌŅņ__________”£
£Ø14·Ö£©£Ø1£©bd£Ø2·Ö£© £Ø2£©¢Łb a£Ø2·Ö£©£»°±ĘųČܽā¶Č“ó£¬ĻČĶØČė°±ĘųŹ¹ČÜŅŗĻŌ¼īŠŌ£¬Ōö“óCO2µÄĪüŹÕĮ棬Ōö“óHCO3£µÄÅØ¶Č£Ø2·Ö£© ¢ŚČ”ŹŹĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČėÅØNaOHČÜŅŗ£¬¼ÓČČ£¬²śÉś“Ģ¼¤ŠŌĘųĪ¶ĘųĢå£ØNH3£©£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£Ø2·Ö£© ¢Ū±„ŗĶĢ¼ĖįĒāÄĘČÜŅŗ
£Ø3£©ŪįŪö”¢ŪįŪöĒÆ£Ø1·Ö£©£ØĪ““š³öŪįŪöĒƲ»øų·Ö£©
£Ø4£©¢ŁB£Ø1·Ö£©£»µķ·Ūµā»Æ¼ŲČÜŅŗ»ņŹŖČóµÄµķ·Ūµā»Æ¼ŲŹŌÖ½£Ø1·Ö£©
¢Śµē½āÉś³ÉµÄĀČĘų»įÓėNaOH³ä·Ö·“Ó¦£¬µ¼ÖĀµē½ā×īÖÕ²śĪļ½öŹĒNaClOŗĶH2”£NaClO¾ßÓŠĒæŃõ»ÆŠŌ£¬½«·ÓĢŖŃõ»Æ£¬¹ŹČÜŅŗ²»±äŗģÉ«£Ø2·Ö£©
”¾½āĪö”æ
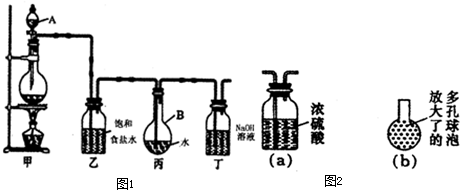
ŹŌĢā·ÖĪö£ŗ£Ø1£©ÓÉÓŚŌŚ³żŌÓŹ±²»ÄÜŌŁŅżČėŠĀµÄŌÓÖŹ£¬ĖłŅŌŅŖ³żČ„Ź³ŃĪČÜŅŗÖŠŗ¬ÓŠSO42£µČŌÓÖŹ£¬æÉŅŌŃ”ŌńĒāŃõ»Æ±µ»ņĀČ»Æ±µ£¬¶ų²»ÄÜŃ”ŌńĻõĖį±µ»ņĢ¼Ėį±µ£¬“š°øŃ”bd”£
£Ø2£©¢Łøł¾Ż×°ÖĆĢŲµćæÉÖŖ£¬A×°ÖĆŹĒÖʱøĢ¼ĖįĒāÄĘµÄ£¬B×°ÖĆŹĒÖʱøCO2µÄ£¬C×°ÖĆŹĒÖʱø°±ĘųµÄ£¬D×°ÖĆŹĒĪüŹÕ¶ąÓąµÄ°±Ęų”£ÓÉÓŚ°±Ęų¼«Ņ×ČÜÓŚĖ®£¬ĖłŅŌ°±ĘųŅŖĶعżaæŚĶØČėµ½×°ÖĆAÖŠ£¬ĖłŅŌÕżČ·Į¬½ÓĖ³ŠņŹĒd½Ób£¬e½Óa£¬c½Óf”£ÓÉÓŚ°±ĘųČܽā¶Č“ó£¬ĻČĶØČė°±ĘųŹ¹ČÜŅŗĻŌ¼īŠŌ£¬Ōö“óCO2µÄĪüŹÕĮ棬“Ó¶ųŌö“óHCO3£µÄÅØ¶Č£¬±ćÓŚĢ¼ĖįĒāÄĘ¾§ĢåĪö³ö”£
¢Śļ§ŃĪÄÜŗĶĒæ¼ī·“Ӧɜ³É°±Ęų£¬æÉŅŌĶعż¼ģŃé°±ĘųĄ“¼ģŃéNH4£«£¬ĖłŅŌÕżČ·²Ł×÷ŹĒČ”ŹŹĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČėÅØNaOHČÜŅŗ£¬¼ÓČČ£¬²śÉś“Ģ¼¤ŠŌĘųĪ¶ĘųĢå£ØNH3£©£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶”£
¢ŪÓÉÓŚŃĪĖįŅ×»Ó·¢£¬ĖłŅŌÉś³ÉµÄCO2ÖŠŗ¬ÓŠĀČ»ÆĒā£¬¶ųĀČ»ÆĒāÄÜŗĶ°±Ęų·“Ó¦£¬ĖłŅŌŠ”ÓŚ³żČ„CO2ÖŠµÄĀČ»ÆĒāĘųĢ壬Ņņ“ĖĘægÄŚµÄČÜŅŗŹĒ±„ŗĶĢ¼ĖįĒāÄĘČÜŅŗ”£
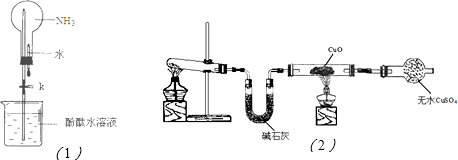
£Ø3£©Ģ¼ĖįĒāÄĘ¼ÓČČ·Ö½ā¼“Éś³ÉĢ¼ĖįÄĘ£¬¶ų¹ĢĢå¼ÓČČŠčŅŖŌŚŪįŪöÖŠ½ųŠŠ£¬ĖłŅŌ»¹Č±ÉŁµÄŅĒĘ÷ŹĒŪįŪöŗĶŪįŪöĒÆ”£
£Ø4£©¢Łøł¾Ż×°ÖĆĶ¼æÉÖŖ£¬AŗĶµēŌ“µÄøŗ¼«ĻąĮ¬£¬×öŅõ¼«£¬ČÜŅŗÖŠµÄĒāĄė×ӷŵēÉś³ÉĒāĘų”£BŗĶµēŌ“µÄÕż¼«ĻąĮ¬£¬×öŃō¼«£¬ČÜŅŗÖŠµÄĀČĄė×ӷŵēÉś³ÉĀČĘų£¬ĖłŅŌĀČĘųŌŚBµē¼«Éś³É”£ĀČĘų¾ßÓŠĒæŃõ»ÆŠŌ£¬ÄÜŹ¹ŹŖČóµÄµķ·Ūµā»Æ¼ŲŹŌÖ½±äĄ¶É«£¬¾Ż“ĖæÉŅŌ¼ģŃéĀČĘų£¬Ņņ“Ė¼ģŃéC12ĖłÓĆŹŌ¼Į£ØÓĆĘ·£©ŹĒµķ·Ūµā»Æ¼ŲČÜŅŗ»ņŹŖČóµÄµķ·Ūµā»Æ¼ŲŹŌÖ½”£
¢ŚÓÉÓŚµē½āÉś³ÉµÄĀČĘų»įÓėNaOH³ä·Ö·“Ó¦£¬Ņņ“Ė³¤Ź±¼äµē½āŗó»įµ¼ÖĀµē½ā×īÖÕ²śĪļ½öŹĒNaClOŗĶH2”£NaClO¾ßÓŠĒæŃõ»ÆŠŌ£¬½«·ÓĢŖŃõ»Æ£¬¹ŹČÜŅŗ²»±äŗģÉ«”£
æ¼µć£ŗæ¼²éĄė×ӵijżŌÓ£»Ģ¼ĖįĒāÄĘ”¢CO2ŗĶ°±ĘųµÄÖʱø£»ŅĒĘ÷µÄŃ”Ōń£»NH4£«ŗĶĀČĘųµÄ¼ģŃ飻µē½ā±„ŗĶŹ³ŃĪĖ®µÄÓŠ¹ŲÅŠ¶ĻµČ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2012?¶«³ĒĒųŅ»Ä££©Ä³ŃŠ¾æŠŌѧĻ°Š”×éŌŚÕūĄķŹµŃéŹŅ»ÆѧŹŌ¼ĮŹ±£¬·¢ĻÖŅ»ĘæŹ¢ÓŠĪŽÉ«ČÜŅŗµÄŹŌ¼Į£¬±źĒ©ĘĘĖš£¬ČēĶ¼£®Ä³Ķ¬Ń§øł¾ŻÖŠŃ§»ÆѧÖŖŹ¶£¬¶ŌøĆČÜŅŗÖŠµÄČÜÖŹ³É·Ö½ųŠŠČēĻĀŌ¤²āŗĶŃéÖ¤£¬ĘäÖŠ“ķĪóµÄŹĒ£Ø°üĄØŌ¤²āĪļÖŹµÄ»ÆѧŹ½”¢¼ģŃéŠčŅŖµÄŹŌ¼Į”¢²Ł×÷”¢ĻÖĻó¼°½įĀŪ£©£Ø””””£© £Ø2012?¶«³ĒĒųŅ»Ä££©Ä³ŃŠ¾æŠŌѧĻ°Š”×éŌŚÕūĄķŹµŃéŹŅ»ÆѧŹŌ¼ĮŹ±£¬·¢ĻÖŅ»ĘæŹ¢ÓŠĪŽÉ«ČÜŅŗµÄŹŌ¼Į£¬±źĒ©ĘĘĖš£¬ČēĶ¼£®Ä³Ķ¬Ń§øł¾ŻÖŠŃ§»ÆѧÖŖŹ¶£¬¶ŌøĆČÜŅŗÖŠµÄČÜÖŹ³É·Ö½ųŠŠČēĻĀŌ¤²āŗĶŃéÖ¤£¬ĘäÖŠ“ķĪóµÄŹĒ£Ø°üĄØŌ¤²āĪļÖŹµÄ»ÆѧŹ½”¢¼ģŃéŠčŅŖµÄŹŌ¼Į”¢²Ł×÷”¢ĻÖĻó¼°½įĀŪ£©£Ø””””£©
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ŹµŃé²½Öč | ½į¹ū·ÖĪö |
| ²½Öč1£ŗȔɣĮæѳʷӌŹŌ¹ÜÖŠ£¬¼ÓČėŹŹĮæÕōĮóĖ®Ź¹Ö®Čܽā£¬ µĪ¼Ó×ćĮæĻ”ĻõĖįĖį»Æ£¬ŌŁµĪ¼Ó¼øµĪAgNO3ČÜŅŗ µĪ¼Ó×ćĮæĻ”ĻõĖįĖį»Æ£¬ŌŁµĪ¼Ó¼øµĪAgNO3ČÜŅŗ £® |
ÓŠ°×É«³ĮµķÉś³É£¬ĖµĆ÷²śĘ·ÖŠŗ¬ÓŠNaCl£® |
| ²½Öč2£ŗĮķȔɣĮæѳʷӌ׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæÕōĮóĖ®Ź¹Ö®Čܽā£¬¼ÓČė¼øµĪ·ÓĢŖ£¬ÓĆ0.1000mol?L-1ŃĪĖįµĪ¶ØÖĮČÜŅŗÓÉŗģÉ«±äĪŽÉ«£¬¼ĒĀ¼ĻūŗÄŃĪĖįµÄĢå»żV1£® ŌŁĻņŅѱäĪŽÉ«µÄČÜŅŗÖŠµĪ¼Ó¼øµĪ¼×»ł³Č£¬¼ĢŠųÓĆøĆŃĪĖįµĪ¶ØÖĮČÜŅŗÓÉ»ĘÉ«±äĪŖ³ČÉ« ŌŁĻņŅѱäĪŽÉ«µÄČÜŅŗÖŠµĪ¼Ó¼øµĪ¼×»ł³Č£¬¼ĢŠųÓĆøĆŃĪĖįµĪ¶ØÖĮČÜŅŗÓÉ»ĘÉ«±äĪŖ³ČÉ« £¬¼ĒĀ¼ĻūŗÄŃĪĖįµÄĢå»żV2£® |
V2£¾V1 V2£¾V1 £¬ĖµĆ÷²śĘ·ÖŠŗ¬ÓŠNaHCO3£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| ||
| ||
 £»
£» £»
£»| H+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com