
ҽ���Ȼ��ƿ����ڲ��ơ������������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƹ�������Ϊ��

��֪���������ϵ�֪�����������ʱ��pHΪ��
|
�������� |
Fe(OH)3 |
Al(OH)3 |
|
|
��ʼ����ʱ��pH |
2.3 |
4.0 |
��ʼ�ܽ⣺7.8 |
|
��ȫ����ʱ��pH |
3.7 |
5.2 |
��ȫ�ܽ⣺10.8 |
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2�����Ӳ����Ǽ����������ƣ�������Һ��pHΪ ��Ŀ���dz�ȥ��Һ��������Al3����Fe3��������Fe(OH)3�Ƿ������ȫ��ʵ�������
��
��3������ʱ���õIJ��������� ��������Ҫ�ɷֵĻ�ѧʽ ��
��4���ữʱ�������Ŀ��Ϊ���� ���ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��5��Ϊʲô�����ᾧҪ������160�棺 ��
��6���ⶨ������Ʒ�Ĵ��ȣ�����һ��Ũ�ȵ�AgNO3��Һ�ζ�һ�������ľ�����Ʒ��������Ʒ��CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
��16�֣�ÿ��2�֣�
��1��CaCO3+2H+=Ca2++CO2��+H2O
��2��5.2~7.8����֮�������ֵ��5.2��pH<7.8�� ���ã�ȡ�����ϲ���Һ��С�Թ��У��μ�KSCN��Һ�������Ժ�ɫ����Fe(OH)3������ȫ����֮����Fe(OH)3��������ȫ
��3) �ձ�������������ͨ©�� Fe(OH)3 ��Al(OH)3
��4����ȥ�������������ƣ����ֹ�����������տ����еĶ�����̼����ֹ������ˮ��ȣ���������֣�
��5���¶�̫��CaCl2 ��2H2O��ʧˮ
��6�� Na+û��ȥ����NaCl�����²ⶨ���ƫ�ߣ�������CaCl2 ��2H2Oʧˮ��
��������
�����������1��������ǿ�ᣬ���Ա�̼��ǿ����������ˮ��̼������������ᣬ���ɿ����Ե��Ȼ��ơ�������̼�����ˮ��������������Ļ������д��������ʽ���������ʶ�������ѧʽ��ɾȥʵ����δ��Ӧ�����ӣ���÷�ӦΪCaCO3+2H+=Ca2++CO2��+H2O����2�����ݱ�������������ȫ����ʱ��pH��֪����ȥ��Һ�����������Ӻ�������Ӧʹ��ҺpH����5.2��7.8֮�����5.2��pH<7.8������3.7ʱ�����Ӳ�����ȫ��Ϊ������������������5.2ʱ�����Ӳ�����ȫ��Ϊ������������������7.8ʱ������������ʼ��Ϊƫ��������ӣ���Һ�������µ����ʣ��������������Ƿ�����ķ������Ǽ�����Һ���Ƿ���������ӣ�������������KSCN��Һ����������Ӧ��ͨ����Ƶ�ʵ�鷽��Ϊ�����ã�ȡ�����ϲ���Һ��С�Թ��У��μ�KSCN��Һ�������Ժ�ɫ����Fe(OH)3������ȫ����֮����Fe(OH)3��������ȫ����3���������ڻ�ѧʵ�����������Ҳ�ǻ����������ᴿ�ij��÷�������Ҫʹ���ձ�������������ͨ©��������������ҺpH����5.2��7.8֮��ʱ�������ӡ���������ȫ��Ϊ������������������������������������������ijɷ�ΪFe(OH)3��Al(OH)3����4�����������dz����Լ��������Լ�һ������������������ǿ�����Ŀ��������Ȼ��ƣ����ữʱ���������Ŀ���dz�ȥ�������������ƣ�����ת��Ϊ�Ȼ��ƣ���ֹ�����������տ����еĶ�����̼���ҹ��������������ӷ��ݳ����ݳ����Ȼ��������ѭ�����ã������ܼ�����������ữ����Ϊ���dz�ȥ������ʱ��������������ӻ���������ӵ������ʣ���5���ᾧˮ���������ȿ���ʧȥ�ᾧˮ�������ᾧҪ�����¶���160���ԭ���ǣ��¶�̫�ߣ���ˮ���Ȼ��ƻ�ʧȥ�ᾧˮ����6���ζ�ԭ��Ϊ��Ag++Cl��=AgCl�����������ӵ����ʵ���ƫ����ⶨ���ƫ�ߣ��ữ��������Һ��Ҫ�����Ȼ��ơ��Ȼ��ơ��Ȼ��⣬�����ᾧʱ�ݳ��Ȼ��⣬����û�г�ȥ�����ӣ������þ����Ƕ�ˮ���Ȼ��ƺ��Ȼ��ƵĻ�����Ԫ�ص�����������CaCl2>NaCl> CaCl2•H2O >CaCl2•2H2O����˾�����Ʒ�к���NaCl���ˮ���Ȼ���ʧȥȫ���ֽᾧˮ�õ���CaCl2��CaCl2•H2O�����ܵ���������Ԫ�ص���������ƫ�ߣ���������Ʒ��ˮ���Ȼ��Ƶ���������ƫ�ߡ�
���㣺�����й������Ʊ��Ļ�ѧ���������ƶ��⣬�漰��Ҫ���������ӷ���ʽ��������ҺpH��ȥ���ʽ��������ӡ������������Ƿ������ȫ�ķ������������������������ɷ֡��ữ��Ŀ�ġ������¶ȵ�ԭ����Ʒ���Ȳⶨ���ƫ�ߵ�ԭ��ȡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ��
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��2012ѧ��㶫ʡ����ѧ���������ܲ⻯ѧ�Ծ� ���ͣ�ʵ����
��16�֣�ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
(�����õ���ԭ������Cl 35.5 Ca 40 O 16 )
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺  ��
��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ����ֻд����ʽ������������
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�ٺ��а�����ѧ������ѧ�ڵ�һ���¿���ѧ����� ���ͣ�ʵ����
��14�֣�ҽ���Ȼ��� �����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
�����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ�� 
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô���� �ᾧҪ������160�棺 ��
�ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ��
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�ٺ��и�����ѧ�ڵ�һ���¿���ѧ����� ���ͣ�ʵ����
��14����ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
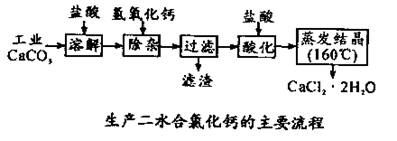
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ��
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ���������ܲ⻯ѧ�Ծ� ���ͣ�ʵ����
��16�֣�ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
(�����õ���ԭ������Cl 35.5 Ca 40 O 16 )

��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ����ֻд����ʽ������������
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com