

| ”÷ |
| ||
| ||
 £¬¹Ź“š°øĪŖ£ŗ3Cu+8H++2NO3?=3Cu2++2NO”ü+4H2O£»ĻõĖį£»31.5g£»1£ŗ1£»3£ŗ2£»
£¬¹Ź“š°øĪŖ£ŗ3Cu+8H++2NO3?=3Cu2++2NO”ü+4H2O£»ĻõĖį£»31.5g£»1£ŗ1£»3£ŗ2£» £»
£»| ”÷ |
| ”÷ |
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø¹² 11 ·Ö£©
£Ø1£©ÓŠŅ»ÖÖ½Š×ö¼Ų³¤ŹÆ£ØK2Al2Si6O16£©£¬Ęä»ÆѧŹ½ĪŖŠ“³ÉŃõ»ÆĪļµÄŠĪŹ½ĪŖ______________
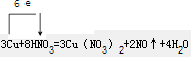
£Ø2£©·“Ó¦3Cu+8HNO3 = 3Cu(NO3)2+2NO”ü+4H2OµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ
ĆæÉś³É11.2L±ź×¼×“æöĻĀµÄĘųĢ壬±»»¹ŌµÄ £ØĢīĆū³Ę£©µÄÖŹĮæĪŖ””””””””g”£
µĆµē×ÓÓėŹ§µē×ÓøöŹż±ČŹĒ_____£¬±»Ńõ»ÆÓė±»»¹ŌµÄŌ×ÓµÄøöŹż±ČĪŖ______£¬ŹŌÓĆ”°µ„ĻßĒÅ”±±ź³öøĆ·“Ó¦µē×Ó×ŖŅĘ·½ĻņŗĶŹżÄæ£ŗ_____________________________
![]() £Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
£Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
¢Ł A + H2O ”ś B + C ¢Ś C + F ”ś D ¢Ū D + NaOH ”ś F + E + H2O
Š“³öA ”¢B”¢ CµÄ»ÆѧŹ½A______ B ____ C______
Š“³ö·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010”Ŗ20111ѧğŗÓ±±Ź”ŗāĖ®ÖŠŃ§øßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø¹² 11 ·Ö£©
£Ø1£©ÓŠŅ»ÖÖ½Š×ö¼Ų³¤ŹÆ£ØK2Al2Si6O16£©£¬Ęä»ÆѧŹ½ĪŖŠ“³ÉŃõ»ÆĪļµÄŠĪŹ½ĪŖ______________
£Ø2£©·“Ó¦3Cu+8HNO3 = 3Cu(NO3)2+2NO”ü+4H2O µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ
ĆæÉś³É11.2L±ź×¼×“æöĻĀµÄĘųĢ壬±»»¹ŌµÄ £ØĢīĆū³Ę£©µÄÖŹĮæĪŖ””””””””g”£
µĆµē×ÓÓėŹ§µē×ÓøöŹż±ČŹĒ_____£¬±»Ńõ»ÆÓė±»»¹ŌµÄŌ×ÓµÄøöŹż±ČĪŖ______£¬ŹŌÓĆ”°µ„ĻßĒÅ”±±ź³öøĆ·“Ó¦µē×Ó×ŖŅĘ·½ĻņŗĶŹżÄæ£ŗ_____________________________ £Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
£Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
¢Ł A + H2O ”ś B + C ¢Ś C + F ”ś D ¢Ū D + NaOH ”ś F + E + H2O
Š“³öA ”¢B”¢ CµÄ»ÆѧŹ½A______ B ____ C______
Š“³ö·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-20111ѧğŗÓ±±Ź”øßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø¹² 11 ·Ö£©
£Ø1£©ÓŠŅ»ÖÖ½Š×ö¼Ų³¤ŹÆ£ØK2Al2Si6O16£©£¬Ęä»ÆѧŹ½ĪŖŠ“³ÉŃõ»ÆĪļµÄŠĪŹ½ĪŖ______________
£Ø2£©·“Ó¦3Cu+8HNO3 = 3Cu(NO3)2+2NO”ü+4H2O µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ
ĆæÉś³É11.2L±ź×¼×“æöĻĀµÄĘųĢ壬±»»¹ŌµÄ £ØĢīĆū³Ę£©µÄÖŹĮæĪŖ””””””””g”£
µĆµē×ÓÓėŹ§µē×ÓøöŹż±ČŹĒ_____£¬±»Ńõ»ÆÓė±»»¹ŌµÄŌ×ÓµÄøöŹż±ČĪŖ______£¬ŹŌÓĆ”°µ„ĻßĒÅ”±±ź³öøĆ·“Ó¦µē×Ó×ŖŅĘ·½ĻņŗĶŹżÄæ£ŗ_____________________________
 £Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
£Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
¢Ł A + H2O ”ś B + C ¢Ś C + F ”ś D ¢Ū D + NaOH ”ś F + E + H2O
Š“³öA ”¢B”¢ CµÄ»ÆѧŹ½A______ B ____ C______
Š“³ö·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø¹² 11 ·Ö£©
£Ø1£©ÓŠŅ»ÖÖ½Š×ö¼Ų³¤ŹÆ£ØK2Al2Si6O16£©£¬Ęä»ÆѧŹ½ĪŖŠ“³ÉŃõ»ÆĪļµÄŠĪŹ½ĪŖ______________
£Ø2£©·“Ó¦3Cu+8HNO3 = 3Cu(NO3)2+2NO”ü+4H2O µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ
ĆæÉś³É11.2L±ź×¼×“æöĻĀµÄĘųĢ壬±»»¹ŌµÄ £ØĢīĆū³Ę£©µÄÖŹĮæĪŖ””””””””g”£
µĆµē×ÓÓėŹ§µē×ÓøöŹż±ČŹĒ_____£¬±»Ńõ»ÆÓė±»»¹ŌµÄŌ×ÓµÄøöŹż±ČĪŖ______£¬ŹŌÓĆ”°µ„ĻßĒÅ”±±ź³öøĆ·“Ó¦µē×Ó×ŖŅĘ·½ĻņŗĶŹżÄæ£ŗ_____________________________
![]() £Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
£Ø3£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
¢Ł A + H2O ”ś B + C ¢Ś C + F ”ś D ¢Ū D + NaOH ”ś F + E + H2O
Š“³öA ”¢B”¢ CµÄ»ÆѧŹ½A______ B ____ C______
Š“³ö·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com