
��11�֣���֪ ��
��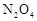 �����ת����
�����ת���� �������������ֽ�һ����
�������������ֽ�һ���� ��
��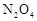 �Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��
�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��
��1��ͼ�й�����������X��Y����������________��ʾ Ũ����ʱ��ı仯������ʵ��X��Y�������߱仯�����У�������
Ũ����ʱ��ı仯������ʵ��X��Y�������߱仯�����У�������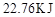 ����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��2��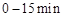 ����Ӧ
����Ӧ ��ƽ�ⳣ��K(b)=__________���Ƚ�
��ƽ�ⳣ��K(b)=__________���Ƚ� ��
�� ʱƽ����Ӧ����
ʱƽ����Ӧ����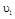 ��
��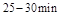 ʱƽ����Ӧ����
ʱƽ����Ӧ���� �Ĵ�С__________��
�Ĵ�С__________��
��3����Ӧ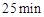 ʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������________�������ֱ������ƽ�ⳣ��K(d)_______K(b)���>������=����<������
ʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������________�������ֱ������ƽ�ⳣ��K(d)_______K(b)���>������=����<������
��4����Ҫ�ﵽʹ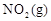 ������ٷֺ�����d����ͬ�Ļ�ѧƽ��״̬����
������ٷֺ�����d����ͬ�Ļ�ѧƽ��״̬����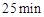 ʱ�����Բ�ȡ�Ĵ�ʩ��___________��
ʱ�����Բ�ȡ�Ĵ�ʩ��___________��
A��������� B����С������� C�������¶� D������һ������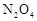
��11�֣���1��X��1�֣�
2NO2 N2O4
��H����56.9KJ/mol��2�֣�
N2O4
��H����56.9KJ/mol��2�֣�
��2��K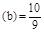 ��
��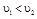 ����2�֣� ��3������
����2�֣� ��3������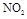 ��Ũ��
=����1�֣���4��BD��2�֣�
��Ũ��
=����1�֣���4��BD��2�֣�
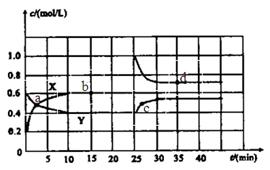
����������1������ͼ���֪������X��Ũ�ȱ仯��������Y��Ũ�ȱ仯����2�������Ը��ݷ���ʽ��֪��X���߱�ʾNO2��Ũ����ʱ��ı仯������ͼ���֪��N2O4��Ũ�ȼ�����0.6mol/L��0.4mol/L��0.2mol/L�����ʵ�����0.4mol����������1molN2O4�ķ�Ӧ����22.76kJ��0.4mol��56.9kJ/mol������Ȼ�ѧ����ʽ��2NO2 N2O4
��H����56.9KJ/mol��
N2O4
��H����56.9KJ/mol��
��2������ͼ���֪��10min��Ӧ�ﵽƽ�⣬N2O4��NO2��Ũ�ȷֱ���0.4mol/L��0.6mol/L�����Է�Ӧ��ƽ�ⳣ���� ������ͼ���֪��0��10min��N2O4�ķ�Ӧ������0.2mol/L��10min��0.02mol/(L��min)����25��30min��N2O4�ķ�Ӧ������0.15mol/L��5min��0.03mol/(L��min)������
������ͼ���֪��0��10min��N2O4�ķ�Ӧ������0.2mol/L��10min��0.02mol/(L��min)����25��30min��N2O4�ķ�Ӧ������0.15mol/L��5min��0.03mol/(L��min)������ ��
��
��3������ͼ���֪��25minʱNO2��Ũ��ͻȻ����N2O4��Ũ�����������Ըı�������������NO2��Ũ�ȣ��¶Ȳ��䣬����ƽ�ⳣ�����䡣
��4���������ܸı�ƽ��״̬��A����ȷ����С�����ݻ���ѹǿ����ƽ��������Ӧ�����ƶ��������¶ȣ�ƽ�����淴Ӧ������У�����һ������N2O4���൱������ѹǿ��ƽ��������Ӧ������У�������ȷ�Ĵ�ѡBD��


 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
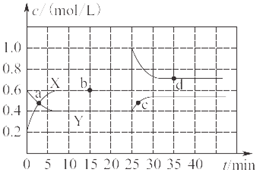 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��| 10 |
| 9 |
| 10 |
| 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
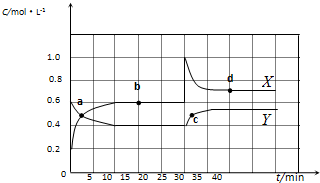 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��| 10 |
| 9 |
| 10 |
| 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡТ�и��и߶�9�µ��п��Ի�ѧ�Ծ����������� ���ͣ������
��11�֣���֪ ��
�� �����ת����
�����ת���� �������������ֽ�һ����
�������������ֽ�һ���� ��
�� �Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��
�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��
��1��ͼ�й�����������X��Y����������________��ʾ Ũ����ʱ��ı仯������ʵ��X��Y�������߱仯�����У�������
Ũ����ʱ��ı仯������ʵ��X��Y�������߱仯�����У������� ����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��2�� ����Ӧ
����Ӧ ��ƽ�ⳣ��K(b)=__________���Ƚ�
��ƽ�ⳣ��K(b)=__________���Ƚ� ��
�� ʱƽ����Ӧ����
ʱƽ����Ӧ���� ��
�� ʱƽ����Ӧ����
ʱƽ����Ӧ���� �Ĵ�С__________��
�Ĵ�С__________��
��3����Ӧ ʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������________�������ֱ������ƽ�ⳣ��K(d)_______K(b)���>������=����<������
ʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������________�������ֱ������ƽ�ⳣ��K(d)_______K(b)���>������=����<������
��4����Ҫ�ﵽʹ ������ٷֺ�����d����ͬ�Ļ�ѧƽ��״̬����
������ٷֺ�����d����ͬ�Ļ�ѧƽ��״̬���� ʱ�����Բ�ȡ�Ĵ�ʩ��___________��
ʱ�����Բ�ȡ�Ĵ�ʩ��___________��
| A��������� | B����С������� | C�������¶� | D������һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪
��֪![]() ��
��![]() �����ת����
�����ת����![]() �������������ֽ�һ����
�������������ֽ�һ����![]() ��
��![]() �Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ����ͼ��
�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ����ͼ��
![]()
![]() ��1��ͼ�й�����������X��Y����������________��ʾ
��1��ͼ�й�����������X��Y����������________��ʾ![]() Ũ����ʱ��ı仯������ʵ��X��Y�������߱仯�����У�������
Ũ����ʱ��ı仯������ʵ��X��Y�������߱仯�����У�������![]() ����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
����������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��2��![]() ����Ӧ
����Ӧ![]() ��ƽ�ⳣ��K(b)=__________���Ƚ�
��ƽ�ⳣ��K(b)=__________���Ƚ�![]() ��
��![]() ʱƽ����Ӧ����
ʱƽ����Ӧ����![]() ��
��![]() ʱƽ����Ӧ����
ʱƽ����Ӧ����![]() �Ĵ�С__________��
�Ĵ�С__________��
��3����Ӧ![]() ʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������________�������ֱ������ƽ�ⳣ��K(d)_______K(b)���>������=����<������
ʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������________�������ֱ������ƽ�ⳣ��K(d)_______K(b)���>������=����<������
��4����Ҫ�ﵽʹ![]() ������ٷֺ�����d����ͬ�Ļ�ѧƽ��״̬����
������ٷֺ�����d����ͬ�Ļ�ѧƽ��״̬����![]() ʱ�����Բ�ȡ�Ĵ�ʩ��___________��
ʱ�����Բ�ȡ�Ĵ�ʩ��___________��
A��������� B�����������
C�������¶� D������һ������![]()
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com