

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĪļĄķ½ĢŃŠŹŅ ĢāŠĶ£ŗ058
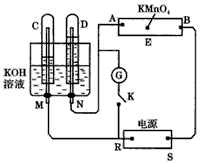
ČēĶ¼ĻĀĖłŹ¾£¬EĪŖÕ“ÓŠ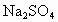 ČÜŅŗµÄĀĖÖ½£¬²¢¼ÓČė¼øµĪ·ÓĢŖ£®A”¢B·Ö±šĪŖPtʬ£¬Ń¹ŌŚĀĖÖ½Į½¶Ė£¬R”¢SĪŖµē³ŲµÄµē¼«£®M”¢NŹĒ¶ąĪ¢æ×µÄNiµÄµē¼«²ÄĮĻ£¬ĖüŌŚ¼īČÜŅŗÖŠæÉŅŌŹÓĪŖ¶čŠŌµē¼«£®GĪŖµēĮ÷¼Ę£¬KĪŖæŖ¹Ų£®C”¢DŗĶµē½ā³ŲÖŠ¶¼³äĀśÅØKOHČÜŅŗ£®ČōŌŚĀĖÖ½ÖŠŃėµćÉĻŅ»µĪ×ĻÉ«µÄ
ČÜŅŗµÄĀĖÖ½£¬²¢¼ÓČė¼øµĪ·ÓĢŖ£®A”¢B·Ö±šĪŖPtʬ£¬Ń¹ŌŚĀĖÖ½Į½¶Ė£¬R”¢SĪŖµē³ŲµÄµē¼«£®M”¢NŹĒ¶ąĪ¢æ×µÄNiµÄµē¼«²ÄĮĻ£¬ĖüŌŚ¼īČÜŅŗÖŠæÉŅŌŹÓĪŖ¶čŠŌµē¼«£®GĪŖµēĮ÷¼Ę£¬KĪŖæŖ¹Ų£®C”¢DŗĶµē½ā³ŲÖŠ¶¼³äĀśÅØKOHČÜŅŗ£®ČōŌŚĀĖÖ½ÖŠŃėµćÉĻŅ»µĪ×ĻÉ«µÄ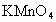 ČÜŅŗ£¬K“ņæŖ£¬½ÓĶصēŌ“Ņ»¶ĪŹ±¼äŗó£¬C”¢DÖŠÓŠĘųĢå²śÉś£®
ČÜŅŗ£¬K“ņæŖ£¬½ÓĶصēŌ“Ņ»¶ĪŹ±¼äŗó£¬C”¢DÖŠÓŠĘųĢå²śÉś£®
(1)ĶāµēŌ“µÄÕż”¢øŗ¼«·Ö±šŹĒRĪŖ___________£¬SĪŖ_________£®
(2)Aø½½üČÜŅŗµÄĻÖĻóŹĒ__________£¬Bø½½ü·¢ÉśµÄµē¼«·“Ó¦Ź½ĪŖ____________£®
(3)ĀĖÖ½ÉĻµÄ×ĻÉ«µćĻņÄÄ·½ŅʶÆ______________________£®
(4)µ±C”¢DĄļµÄĘųĢå²śÉśµ½Ņ»¶ØĮæŹ±£¬ĒŠ¶ĻĶāµēŌ“²¢½ÓĶØæŖ¹ŲK£¬¾¹żŅ»¶ĪŹ±¼ä£¬C”¢DÖŠĘųĢåÖš½„¼õÉŁ£¬Ö÷ŅŖŹĒŅņĪŖ_____________£¬ÓŠ¹ŲµÄ·“Ó¦Ź½ĪŖ______________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ022
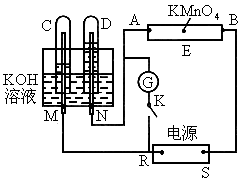
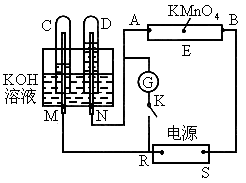
ČēĶ¼ĻĀĖłŹ¾£¬EĪŖÕ“ÓŠ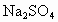 ČÜŅŗµÄĀĖÖ½£¬²¢¼ÓČė¼øµĪ·ÓĢŖ£®A”¢B·Ö±šĪŖPtʬ£¬Ń¹ŌŚĀĖÖ½Į½¶Ė£¬R”¢SĪŖµē³ŲµÄµē¼«£®M”¢NŹĒ¶ąĪ¢æ×µÄNiµÄµē¼«²ÄĮĻ£¬ĖüŌŚ¼īČÜŅŗÖŠæÉŅŌŹÓĪŖ¶čŠŌµē¼«£®GĪŖµēĮ÷¼Ę£¬KĪŖæŖ¹Ų£®C”¢DŗĶµē½ā³ŲÖŠ¶¼³äĀśÅØKOHČÜŅŗ£®ČōŌŚĀĖÖ½ÖŠŃėµćÉĻŅ»µĪ×ĻÉ«µÄ
ČÜŅŗµÄĀĖÖ½£¬²¢¼ÓČė¼øµĪ·ÓĢŖ£®A”¢B·Ö±šĪŖPtʬ£¬Ń¹ŌŚĀĖÖ½Į½¶Ė£¬R”¢SĪŖµē³ŲµÄµē¼«£®M”¢NŹĒ¶ąĪ¢æ×µÄNiµÄµē¼«²ÄĮĻ£¬ĖüŌŚ¼īČÜŅŗÖŠæÉŅŌŹÓĪŖ¶čŠŌµē¼«£®GĪŖµēĮ÷¼Ę£¬KĪŖæŖ¹Ų£®C”¢DŗĶµē½ā³ŲÖŠ¶¼³äĀśÅØKOHČÜŅŗ£®ČōŌŚĀĖÖ½ÖŠŃėµćÉĻŅ»µĪ×ĻÉ«µÄ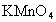 ČÜŅŗ£¬K“ņæŖ£¬½ÓĶصēŌ“Ņ»¶ĪŹ±¼äŗó£¬C”¢DÖŠÓŠĘųĢå²śÉś£®
ČÜŅŗ£¬K“ņæŖ£¬½ÓĶصēŌ“Ņ»¶ĪŹ±¼äŗó£¬C”¢DÖŠÓŠĘųĢå²śÉś£®
(1)ĶāµēŌ“µÄÕż”¢øŗ¼«·Ö±šŹĒRĪŖ___________£¬SĪŖ_________£®
(2)Aø½½üČÜŅŗµÄĻÖĻóŹĒ__________£¬Bø½½ü·¢ÉśµÄµē¼«·“Ó¦Ź½ĪŖ____________£®
(3)ĀĖÖ½ÉĻµÄ×ĻÉ«µćĻņÄÄ·½ŅʶÆ______________________£®
(4)µ±C”¢DĄļµÄĘųĢå²śÉśµ½Ņ»¶ØĮæŹ±£¬ĒŠ¶ĻĶāµēŌ“²¢½ÓĶØæŖ¹ŲK£¬¾¹żŅ»¶ĪŹ±¼ä£¬C”¢DÖŠĘųĢåÖš½„¼õÉŁ£¬Ö÷ŅŖŹĒŅņĪŖ_____________£¬ÓŠ¹ŲµÄ·“Ó¦Ź½ĪŖ______________£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com