
¹¤ŅµÉĻµē½ā±„ŗĶŹ³ŃĪĖ®ÄÜÖĘČ”¶ąÖֻƹ¤ŌĮĻ£¬ĘäÖŠ²æ·ÖŌĮĻæÉÓĆÓŚÖʱø¶ą¾§¹č”£
(1)ÉĻĶ¼ŹĒĄė×Ó½»»»Ä¤·Øµē½ā±„ŗĶŹ³ŃĪĖ®Ź¾ŅāĶ¼£¬µē½ā²ŪŃō¼«²śÉśµÄĘųĢåŹĒ________£»NaOHČÜŅŗµÄ³öæŚĪŖ________(Ģī×ÖÄø)£»¾«Öʱ„ŗĶŹ³ŃĪĖ®µÄ½ųæŚĪŖ________(Ģī×ÖÄø)£»øÉŌļĖžÖŠÓ¦Ź¹ÓƵÄŅŗĢåŹĒ________”£
(2)¶ą¾§¹čÖ÷ŅŖ²ÉÓĆSiHCl3»¹Ō¹¤ŅÕÉś²ś£¬Ęäø±²śĪļSiCl4µÄ×ŪŗĻĄūÓƏܵ½¹ć·ŗ¹Ų×¢”£
¢ŁSiCl4æÉÖĘĘųĻą°×ĢæŗŚ(Óė¹āµ¼ĻĖĪ¬Ö÷ŅŖŌĮĻĻąĶ¬)£¬·½·ØĪŖøßĪĀĻĀSiCl4ÓėH2ŗĶO2·“Ó¦£¬²śĪļÓŠĮ½ÖÖ£¬»Æѧ·½³ĢŹ½ĪŖ___________________________________”£
¢ŚSiCl4æÉ×Ŗ»ÆĪŖSiHCl3¶ųŃ»·Ź¹ÓĆ£¬Ņ»¶ØĢõ¼žĻĀ£¬ŌŚ20 LŗćČŻĆܱÕČŻĘ÷ÖŠµÄ·“Ó¦£ŗ
3SiCl4(g)£«2H2(g)£«Si(s) 4SiHCl3(g)
4SiHCl3(g)
“ļĘ½ŗāŗó£¬H2ŗĶSiHCl3ĪļÖŹµÄĮæÅØ¶Č·Ö±šĪŖ0.140 mol/LŗĶ0.020 mol/L£¬ČōH2Č«²æĄ“Ō“ÓŚĄė×Ó½»»»Ä¤·ØµÄµē½ā²śĪļ£¬ĄķĀŪÉĻŠčĻūŗÄ“æNaClµÄÖŹĮæĪŖ________kg”£
(3)²ÉÓĆĪŽÄ¤µē½ā²Ūµē½ā±„ŗĶŹ³ŃĪĖ®£¬æÉÖĘČ”ĀČĖįÄĘ£¬Ķ¬Ź±Éś³ÉĒāĘų£¬ĻÖÖʵĆĀČĖįÄĘ213.0 kg£¬ŌņÉś³ÉĒāĘų________m3(±ź×¼×“æö)”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ķ¼ĪŖĻą»„“®ĮŖµÄ¼×”¢ŅŅĮ½µē½ā³Ų£®ŹŌ»Ų“š£ŗ
£Ø1£©Čō¼×µē½ā³ŲĄūÓƵē½āŌĄķŌŚĢśÉĻ¶ĘŅų£¬ŌņAŹĒ £ØĢīµē¼«²ÄĮĻ£©£¬µē¼«·“Ó¦Ź½ £»B£ØŅŖĒóĶ¬A£©ŹĒ £¬µē¼«·“Ó¦Ź½ £»Ó¦Ń”ÓƵĵē½āÖŹČÜŅŗŹĒ ”£
£Ø2£©ŅŅµē½ā³ŲÖŠČōµĪČėÉŁĮæ·ÓĢŖŹŌŅŗ£¬æŖŹ¼µē½āŅ»¶ĪŹ±¼ä£¬Ģś¼«ø½½ü³Ź £¬C¼«ø½½ü³Ź ”£
£Ø3£©Čō¼×µē½ā³ŲŅõ¼«ŌöÖŲ4.32g£¬ŌņŅŅ²ŪÖŠŃō¼«ÉĻ·Å³öµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ ”£
£Ø4£©ČōŅŅµē½ā³ŲÖŠŹ£ÓąČÜŅŗČŌĪŖ400mL£¬Ōņµē½āŗóĖłµĆČÜŅŗÖŠŠĀÉś³ÉČÜÖŹµÄĪļÖŹµÄĮæÅضČĪŖ mol?L-1£¬ČÜŅŗµÄpHµČÓŚ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĪŖ³ÖŠųµē½āŗ¬ÓŠŅ»¶ØĮæCaCl2Ė®ČÜŅŗ(ŗ¬·ÓĢŖ)µÄ×°ÖĆ(ŅŌ²¬ĪŖµē¼«),AĪŖµēĮ÷±ķ”£µē½āŅ»¶ĪŹ±¼ät1ŗó,½«CO2Į¬ŠųĶØČėµē½āŅŗÖŠ”£
(1)µē½āŹ±,F¼«·¢Éś””””””””””””·“Ó¦,µē¼«·“Ó¦ĪŖ””””””””””””””,E¼«·¢Éś””””””””””””·“Ó¦,µē¼«·“Ó¦ĪŖ””””””””””””””””””””””,µē½ā×Ü·“Ó¦ĪŖ”””””””””””””””””””””””””£
(2)µē½ā³ŲÖŠ²śÉśµÄĻÖĻó:
¢Ł””_____________________
¢Ś””_____________________
¢Ū””_____________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ń£®¶žŃõ»ÆĀČ£ØClO2£©ŹĒŅ»ÖÖ»ĘĀĢÉ«ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬ĘäČŪµćĪŖ£59”ę£¬·ŠµćĪŖ11.0”ę£¬Ņ×ČÜÓŚĖ®”£¹¤ŅµÉĻÓĆÉŌ³±ŹŖµÄKClO3ŗĶ²ŻĖį£ØH2C2O4£©ŌŚ60”ꏱ·“Ó¦ÖʵƔ£Ä³Ń§ÉśÄāÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÄ£Äā¹¤ŅµÖĘČ”²¢ŹÕ¼ÆClO2”£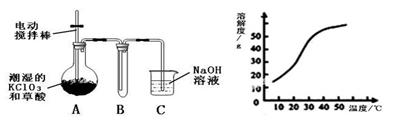
£Ø1£©A±ŲŠėĢķ¼ÓĪĀ¶ČæŲÖĘ×°ÖĆ£¬³ż¾Ę¾«µĘ”¢ĪĀ¶Č¼ĘĶā£¬»¹ŠčŅŖµÄ²£Į§ŅĒĘ÷ÓŠ ”£
£Ø2£©·“Ó¦ŗóŌŚ×°ÖĆCÖŠæɵĆNaClO2ČÜŅŗ”£ŅŃÖŖŌŚĪĀ¶ČµĶÓŚ38”ꏱNaClO2±„ŗĶČÜŅŗÖŠĪö³ö¾§ĢåŹĒNaClO2”¤3H2O£¬ŌŚĪĀ¶ČøßÓŚ38”ꏱĪö³ö¾§ĢåŹĒNaClO2”£øł¾ŻÉĻÓŅĶ¼ĖłŹ¾µÄNaClO2µÄČܽā¶ČĒśĻߣ¬Ēė²¹³ä“ÓNaClO2ČÜŅŗÖŠÖʵĆNaClO2¾§ĢåµÄ²Ł×÷²½Öč£ŗ ¢Ł Õō·¢½į¾§£»¢Ś £»¢Ū Ļ“µÓ£»¢Ü øÉŌļ”£
£Ø3£©ClO2ŗܲ»ĪČ¶Ø£¬ŠčĖęÓĆĖęÖĘ£¬ÓĆĖ®ĪüŹÕµĆµ½ClO2ČÜŅŗ”£ĪŖ²ā¶ØĖłµĆČÜŅŗÖŠClO2µÄÅØ¶Č£¬½ųŠŠĮĖĻĀĮŠŹµŃé£ŗ¢Ł ×¼Č·ĮæČ”ClO2ČÜŅŗV1mL¼ÓČėµ½×¶ŠĪĘæÖŠ£¬¼ÓŹŹĮæÕōĮóĖ®Ļ”ŹĶ£¬µ÷½ŚŹŌŃłµÄpH”Ü2.0”£¢Ś ¼ÓČė×ćĮæµÄKI¾§Ģ壬¾²ÖĆʬæĢ”£“ĖŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ £»¢Ū ¼ÓČėµķ·ŪÖøŹ¾¼Į£¬ÓĆc mol/L Na2S2O3ČÜŅŗµĪ¶Ø£¬ÖĮÖÕµćŹ±ĻūŗÄNa2S2O3ČÜŅŗV2 mL”£ŌņŌClO2ČÜŅŗµÄÅضČĪŖ mol£ÆL£ØÓĆŗ¬×ÖÄøµÄ“śŹżŹ½±ķŹ¾£©”££ØŅŃÖŖ2 Na2S2O3+I2= Na2S4O6+2NaI£©
¢ņ£®½«ÓÉNa+”¢Ba2+”¢Cu2+”¢SO42£”¢Cl£ ×éŗĻŠĪ³ÉµÄČżÖÖĒæµē½āÖŹČÜŅŗ£¬·Ö±š×°ČėĻĀĶ¼×°ÖĆ
ÖŠµÄ¼×”¢ŅŅ”¢±ūČżøöÉÕ±ÖŠ½ųŠŠµē½ā£¬µē¼«¾łĪŖŹÆÄ«µē¼«”£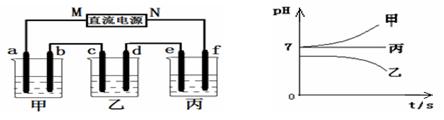
½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£³£ĪĀĻĀø÷ÉÕ±ÖŠČÜŅŗpHÓėµē½āŹ±¼ätµÄ¹ŲĻµČēÓŅÉĻĶ¼£ØŗöĀŌŅņĘųĢåČܽā“ųĄ“µÄÓ°Ļģ£©”£¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öŅŅÉÕ±ÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø2£©µē¼«fÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ £»
£Ø3£©Čō¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆŅŅÉÕ±ÖŠcµē¼«ÖŹĮæŌö¼ÓĮĖ8g£¬ŅŖŹ¹±ūÉÕ±ÖŠČÜŅŗ»Öø“µ½ŌĄ“µÄדĢ¬£¬Ó¦½ųŠŠµÄ²Ł×÷ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅųĘ÷ĆóČÕ¾Ć±ķĆę±äŗŚ,ÕāŹĒÓÉÓŚÉś³ÉĮņ»ÆŅų,ÓŠČĖÉč¼ĘÓĆŌµē³ŲŌĄķ¼ÓŅŌ³żČ„,Ęä·½·ØŹĒ:½«Ņ»¶ØÅØ¶ČµÄŹ³ŃĪČÜŅŗ·ÅČėŅ»ĀĮÖĘČŻĘ÷ÖŠ,ŌŁ½«±äŗŚµÄŅųĘ÷½žČėČÜŅŗÖŠ,·ÅÖĆŅ»¶ĪŹ±¼ä,ŗŚÉ«»įĶŹČ„¶ųŅų²»»įĖšŹ§”£ŹŌ»Ų“š:ŌŚŌµē³Ų·“Ó¦ÖŠ,øŗ¼«·¢ÉśµÄ·“Ó¦ĪŖ £»Õż¼«·¢ÉśµÄ·“Ó¦ĪŖ £»·“Ó¦¹ż³ĢÖŠÓŠ³ō¼¦µ°ĘųĪ¶ĘųĢå²śÉś,ŌņŌµē³ŲµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijæĪĶā»ī¶ÆŠ”×éÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬µ÷½Ś»¬¶Æ±ä×čĘ÷æŲÖʵēĮ÷Ēæ¶ČŹŹÖŠµÄĒéæöĻĀÓĆĘä½ųŠŠ»ŗĀżµē½āNaClČÜŅŗ¼°Ļą¹ŲŹµŃé(“ĖŹ±£¬“ņæŖÖ¹Ė®¼Ša£¬¹Ų±ÕÖ¹Ė®¼Šb)”£ÓÉÓŚ“ÖŠÄ£¬ŹµŃé²¢Ī““ļµ½Ō¤ĘŚÄæµÄ£¬µ«Ņ²æ“µ½ĮĖŗÜĮīČĖøߊĖµÄĻÖĻó”£(ŃōĄė×Ó½»»»Ä¤Ö»ŌŹŠķŃōĄė×ÓŗĶĖ®Ķعż)
Ēė°ļÖśĖūĆĒ·ÖĪö²¢»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öB×°ÖĆÖŠµÄµē¼«·“Ó¦£ŗ
Ņõ¼«£ŗ________£»Ńō¼«£ŗ________”£
(2)¹Ū²ģµ½A×°ÖĆÖŠµÄĻÖĻóŹĒ£ŗ¢Ł________£»¢Ś________£»¢Ū________”£
(3)µ±¹Ū²ģµ½A×°ÖĆÖŠµÄĻÖĻóŗó£¬ĖūĆĒ¹Ų±ÕÖ¹Ė®¼Ša£¬“ņæŖÖ¹Ė®¼Šb”£ŌŁ¹Ū²ģC×°ÖĆ£¬ČōĪŽĻÖĻó£¬ĒėĖµĆ÷ĄķÓÉ£»ČōÓŠĻÖĻó£¬ĒėŠ“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½(ŹĒĄė×Ó·“Ó¦µÄŠ“Ąė×Ó·½³ĢŹ½)£ŗ____________________________________”£
(4)ČōĻė“ļµ½µē½āNaClČÜŅŗµÄÄæµÄ£¬Ó¦ČēŗĪøĽų×°ÖĆ£¬ĒėĢį³öÄćµÄŅā¼ū£ŗ
__________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ģ«ŃōÄܵē³ŲæÉÓĆ×öµē½āµÄµēŌ“(ČēĶ¼)”£
(1)Čōc”¢d¾łĪŖ¶čŠŌµē¼«£¬µē½āÖŹČÜŅŗĪŖĮņĖįĶČÜŅŗ£¬µē½ā¹ż³ĢÖŠ£¬c¼«ĻČĪŽĘųĢå²śÉś£¬ŗóÓÖÉś³ÉĘųĢ壬Ōņc¼«ĪŖ________¼«£¬ŌŚµē½ā¹ż³ĢÖŠ£¬ČÜŅŗµÄpH________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)£¬Ķ£Ö¹µē½āŗó£¬ĪŖŹ¹ČÜŅŗ»Öø“ÖĮŌČÜŅŗÓ¦¼ÓČėŹŹĮæµÄ________”£
(2)Čōc”¢d¾łĪŖĶµē¼«£¬µē½āÖŹČÜŅŗĪŖĀČ»ÆÄĘČÜŅŗ£¬Ōņµē½āŹ±£¬ČÜŅŗÖŠĀČĄė×ÓµÄĪļÖŹµÄĮ潫________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
(3)ČōÓĆŹÆÄ«”¢Ģś×÷µē¼«²ÄĮĻ£¬æÉ×é×°³ÉŅ»øö¼ņŅ×ĪŪĖ®“¦Ąķ×°ÖĆ”£ĘäŌĄķŹĒ£ŗ¼ÓČėŹŌ¼Įµ÷½ŚĪŪĖ®µÄpHŌŚ5.0”«6.0Ö®¼ä”£½ÓĶصēŌ“ŗó£¬Ņõ¼«²śÉśµÄĘųĢ彫ĪŪĪļ“ųµ½Ė®ĆęŠĪ³Éø”Ōü¶ų¹ĪČ„£¬Ęšµ½ø”Ń”¾»»Æ×÷ÓĆ£»Ńō¼«²śÉśµÄÓŠÉ«³Įµķ¾ßÓŠĪüø½ŠŌ£¬Īüø½ĪŪĪļ¶ų³Į»ż£¬Ęšµ½Äż¾Ū¾»»Æ×÷ÓĆ”£øĆ×°ÖĆÖŠ£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ__________________________£»Ńō¼«ĒųÉś³ÉµÄÓŠÉ«³ĮµķŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
”¾ŹµŃéÄæµÄ”æĄūÓĆĖłŃ§ÖŖŹ¶£¬Éč¼Ęµē³Ų×°ÖĆ”£
”¾ŹµŃéÓĆĘ·”æµē¼«£ŗĆ¾Ģõ”¢Ķʬ”¢ĢśĘ¬µČ”£
”¾µē½āÖŹ”æ¹ū֣سČÖ”¢Ę»¹ūÖ”¢ÄūĆŹÖµČ£©”£
”¾ĘäĖū”æµ¼Ļß”¢½šŹō¼Š”¢·¢¹ā¶ž¼«¹Ü”¢500 mLÉÕ±”£
”¾ŹµŃé·½°ø”æ¢ŁCu”ŖMgŌµē³Ų£¬µē½āÖŹČÜŅŗĪŖ³ČÖ£»
¢ŚCu”ŖFeŌµē³Ų£¬µē½āÖŹČÜŅŗĪŖĘ»¹ūÖ£»
¢ŪFe”ŖMgŌµē³Ų£¬µē½āÖŹČÜŅŗĪŖÄūĆŹÖ”£
”¾ŹµŃé²Ł×÷”æÓƵ¼Ļß·Ö±š½«ČżÖÖ·½°øÖŠµÄ½šŹōʬĮ¬½Óµ½½šŹō¼ŠÉĻ£¬·Ö±š½«½šŹōʬĮ½Į½²åČėµ½Ź¢ÓŠ¹ūÖµÄČżøö500 mLµÄÉÕ±ÖŠ£¬ÓĆ·¢¹ā¶ž¼«¹ÜĮ½¶Ė·Ö±š½Ó“„ČżÖÖ·½°øÖŠ½šŹō»ī¶ÆŠŌ²»Ķ¬µÄ½šŹō¼Š”£¹Ū²ģĻÖĻó£¬Į¬½Ó·½Ź½ČēĶ¼ĖłŹ¾”£
”¾ŹµŃéĻÖĻó”æČżÖÖ·½°øÖŠ·¢¹ā¶ž¼«¹Ü¾ł·¢¹ā”£
”¾ŹµŃé½įĀŪ”æŌµē³Ų°Ń»ÆѧÄÜ×Ŗ±äĪŖµēÄÜ”£
»Ų“šĪŹĢā£ŗ
£Ø1£©Į¬½Ó×°ÖĆŹ±»īĘĆ½šŹō½Ó¶ž¼«¹ÜµÄ________¼«ÉĻ£¬½Ļ²»»īĘĆ½šŹō½Ó¶ž¼«¹ÜµÄ________¼«ÉĻ”£
£Ø2£©ŌŚ·½°ø¢Ł¢ŚÖŠĶ×÷µē¼«Ēéæö__________________________”£
£Ø3£©ŌŚ·½°ø¢Ł¢ŪÖŠĆ¾×÷µē¼«Ēéæö__________________________”£
£Ø4£©ŌŚ·½°ø¢Ś¢ŪÖŠĢś×÷µē¼«Ēéæö__________________________”£
£Ø5£©ŌŚ·½°ø¢ŪÖŠøŗ¼«·“Ó¦ĪŖ______£¬Õż¼«·“Ó¦ĪŖ________£¬×Ü·½³ĢŹ½ĪŖ__________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ŲŹÕµÄ·Ļ¾ÉŠæĆĢøɵē³Ų¾¹ż“¦ĄķŗóµĆµ½ĆĢ·Ū(ŗ¬MnO2”¢Mn(OH)2”¢Fe”¢ŅŅČ²ŗĶŗŚĢæµČ)£¬ÓÉĆĢ·ŪÖĘČ”MnO2µÄ²½ÖčČēĻĀĶ¼ĖłŹ¾”£
øł¾ŻÉĻĶ¼ĖłŹ¾²½Öč²¢²Īæ¼±ķøńŹż¾Ż£¬»Ų“šĻĀĮŠĪŹĢā”£
| Īļ ÖŹ | æŖŹ¼³Įµķ | ³ĮµķĶźČ« |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Mn(OH)2 | 8.3 | 9.8 |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com