���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����4.1g�������������������ʵ���Ʒ�����250mL������Һ������ʱ��Ʒ�ɷ���
��������ĸ�� �ϳ���
��A��С�ձ� ��B���ྻֽƬ ��C��ֱ�ӷ���������
��2���ζ�ʱ��һ�㲻��ѡ��
��������ĸ����ָʾ����
��A������ ��B��ʯ�� ��C����̪
��3�������йصζ�������˳����ȷ����
�ټ��ζ����Ƿ�©ˮ ��������ˮϴ�Ӳ������� ���ñ���Һ��ϴʢ����Һ�ĵζ��ܣ��ô���Һ��ϴʢ����Һ�ĵζ��� ��װ����Һ�ʹ���Һ������Һ�棨��¼�������� ��ȡһ������Ĵ���Һ����ƿ�� �ζ�����
��4����0.2010mol?L
-1������ζ������ռ���Һ���ζ�����ȷ����������
��
��5����������ѡ���ָʾ������ȷ�жϵζ��յ��������
��6�������±����ݣ����㱻���ռ�Ũ��Ϊ
���ռ���Ʒ�Ĵ���Ϊ
��
| �ζ����� | ������Һ�����ml�� | �����������ml�� |
| �ζ�ǰ�̶ȣ�ml�� | �ζ���̶ȣ�ml�� |
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 0.20 | 20.80 |
| ������ | 10.00 | 4.10 | 24.00 |
��7������ʵ�������Եζ���������ĺ�������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����
��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����
��





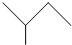
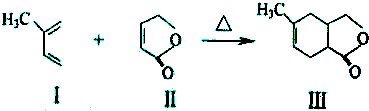
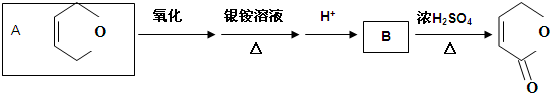
 ��CH��CH����Ȳ��Ҳ�ܷ���Diels-Alder��Ӧ����д���÷�Ӧ����Ľṹ��ʽ
��CH��CH����Ȳ��Ҳ�ܷ���Diels-Alder��Ӧ����д���÷�Ӧ����Ľṹ��ʽ