
 �Ȼ������Ʊ����߷��ӻ����������л��ϳ����й㷺����;��
�Ȼ������Ʊ����߷��ӻ����������л��ϳ����й㷺����;������ ��1���Ȼ���ˮ�������ԣ�
��2��a��AlCl3��Na2CO3����˫ˮ�⣻
b��AlCl3�������������Ʒ�Ӧ����NaAlO2��Һ��
c��AlCl3��NaAlO2����˫ˮ�⣻
d��AlCl3�������Ӧ��
��3�����Թ��еμ���ҺʱӦ����ֱ�����ա������Թܼмг������Թ��ھƾ����ϼ���ʱ��Ҫ�����ƶ��Թܷ�ֹ�ֲ����У�AlCl3��Һ����ʱˮ�ⱻ�ٽ�����Al��OH��3��
��4���ж��Լ��ѹ����ķ����Ǽ����μӣ�����ˮ�������ʱ��ȥ�ϲ���Һ�����μӰ�ˮ�����������ɣ������õ�Al��OH��3������������2-3�Σ����������������0.1g��˵��Al��OH��3�ֽ���ȫ��Al2O3����ѡ��ⶨ����Al��OH��3�����������DzⶨAl2O3��������ԭ����Al��OH��3���ȶ��Բ���Al2O3�ã�
��5���ζ��ܵ�0�̶����ϣ�����0.1120mol HNO3������������ԭ��3.490gAl2��OH��nCl��6-n����OH-���ĵĺͱ�0.1290mol/L�ı�NaOH��Һ���ĵģ��ݴ˼��㣮
��� �⣺��1���Ȼ���ˮ�������ԣ�����������������ˮ�⣬�ʴ�Ϊ����ֹ�Ȼ���ˮ�⣻
��2��a��AlCl3��Na2CO3����˫ˮ�⣺2AlCl3+3Na2CO3+3H2O=2Al��OH��3��+3CO2�����ò���������Һ����a����
b��AlCl3�������������Ʒ�Ӧ����NaAlO2��Һ��AlCl3+4NaOH=3NaCl+NaAlO2+2H2O���ó�����Һ����b��ȷ��
c��AlCl3��NaAlO2����˫ˮ�⣺AlCl3+3NaAlO2+6H2O=4Al��OH��3��+3NaCl���ò���������Һ����c����
d��AlCl3�������Ӧ������Һ��Ϊ���壬��d��ȷ��
��ѡbd��
��3�����Թ��еμ���ҺʱӦ����ֱ�����ա���Ŀ���Ƿ�ֹ��Ⱦ�Լ������Թܼмг������Թ��ھƾ����ϼ���ʱ��Ҫ�����ƶ��Թܷ�ֹ�ֲ����У�AlCl3��Һ����ʱ����ˮ�����ɵ�HCl�ǻӷ����ᣬHCl�Ļӷ�����ˮ�ⱻ�ٽ��������յ�Al��OH��3��
�ʴ�Ϊ����ֹ�Լ�����Ⱦ����ֹ�ֲ��������𱩷У�Al��OH��3��
��4���ж��Լ��ѹ����ķ����Ǽ����μӣ�������ˮ�������ʱ��ȥ�ϲ���Һ�����μӰ�ˮ�����������ɣ������õ�Al��OH��3������������2-3�Σ����������������0.1g��˵��Al��OH��3�ֽ���ȫ��Al2O3����ѡ��ⶨ����Al��OH��3�����������DzⶨAl2O3��������ԭ���Ǹ���Al��OH��3ʱ�ֽ�ʧˮ������Al2O3��ʧˮ���ʴ�Ϊ�����ã�ȡ�ϲ���Һ���μӰ�ˮ���������ɣ�2��ad��
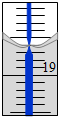
��5���ζ��ܵ�0�̶����ϣ��ʵζ��ܵĶ���Ϊ18.60mL�������ĵ��������Ƶ����Ϊ18.60mL������0.1120mol HNO3������������ԭ��3.490gAl2��OH��nCl��6-n����OH-���ĵĺͱ�0.1290mol/L�ı�NaOH��Һ���ĵģ����У�0.112mol=$\frac{3.490g}{��267-18.5n��g/mol}��n+0.129mol/L��0.0186L��5$��
���n=5��
�ʴ�Ϊ��18.60��5��
���� ���⿼���������仯��������ʺ�����к͵ζ����й����ݣ��ۺ��Խ�ǿ��ע�������������ݵĴ�����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu | B�� | S | C�� | CuS | D�� | Cu2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | n��FeO����n��Fe3O4��=1��1 | B�� | n��Fe2O3����n��FeO��=2��1 | ||
| C�� | n��Fe2O3����n��FeO��=1��2 | D�� | n��Fe2O3����n��Fe3O4��=1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol������ȫȼ������Һ̬ˮ�ų�������С��241.8KJ | |
| B�� | 1molˮ������ȫ�ֽ��������������������241.8kJ���� | |
| C�� | 2mol������1mol������������С��2mol ˮ������������ | |
| D�� | 2mol�������1mol�������������ĵ���������4mol�������ɼ����ų������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | TiCl4���������������ǻ�ԭ���� | |
| B�� | �������뻹ԭ�������ʵ���֮��Ϊ1��1 | |
| C�� | ��ת�Ƶ�����ĿΪ0.2NAʱ�������������1.12L | |
| D�� | ����26g����μӷ�Ӧʱ��ת�Ƶ�����ĿΪNA�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �þƾ���ϴ�������������ձ� | |
| B�� | ��ˮ��ϴ����������Ӧ���Թ� | |
| C�� | ��Ũ������ϴ����������طֽ�ʵ����Թ� | |
| D�� | ������������Һ��ϴʢ�����ӵ��Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����һС��ͭƬ | B�� | ���õ���� 98%������ | ||
| C�� | �õ������۴�����Ƭ | D�� | ���õ����3mol/L���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com