
(12��)����ɸ���������ʵķ����ᴿ��ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�
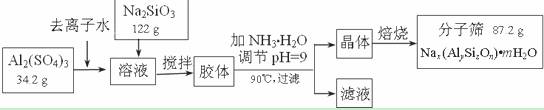
|
��1���ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)=���� �� ��
��2������������������Һ����Ҫ�ɷ�Ϊ���� �� ��д��ѧʽ����
��3������������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10������ͨ������ȷ���÷���ɸ�Ļ�ѧʽ��д��������̣���
��4������ɸ�Ŀ�ֱ��Ϊ4Å��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч���������飨����ֱ��Ϊ4.65Å�����춡�飨����ֱ��Ϊ5.6Å��Ӧ��ѡ������ �� �͵ķ���ɸ��
(12��)
��1��0.001 mol��L��1��2�֣� ��2��Na2SO4��(NH4)2SO4 ��2�֣�
��3�� n[Al2(SO4)3] = 34.2 g / 342 g��mol-1 = 0.1 mol��n(Na2SiO3) = 122 g / 122 g��mol-1 = 1 mol n(Al2O3) = 0.1 mol��n(SiO2) = 1 mol��n(Na2O) = 0.1 mol��n (H2O) = 0.6 mol��3�֣�
n(Na2O) ��n(Al2O3) ��n(SiO2) ��n(H2O) = 0.1 mol��0.1 mol�� 1mol��0.6 mol == 1��1��10��6
��ѧʽ��Na2O ��Al2O3 ��10SiO2 ��6H2O��Na2(Al2Si10O24)��6H2O��3�֣�
��4��5A��2�֣�


 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ʵ���ҿ������̿���Ҫ�ɷ�ΪMnO2���Ʊ�KMnO4���������£����̿����������KOH��KClO3�ڸ����·�Ӧ����������أ�K2MnO4����KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2 ��KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4����ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ��
������������������������������������������������������������������������
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ������������������������������������
��3������2.5g���̿�MnO280������������ʵ�飬����KMnO4�����۲�����
��4��KMnO4�����ȵľ������ữ��Na2C2O4��Ӧ����Mn2+��CO2���÷�Ӧ�Ļ�ѧ����ʽ������������������������������������������������������������������������
��5�������Ƶõ�KMnO4��Ʒ0.165g��ǡ����0.335g��Na2C2O4��Ӧ��ȫ�������KMnO4�Ĵ��ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����и�����ѧ�����п��Ի�ѧ ���ͣ������
(12��)����ɸ���������ʵķ����ᴿ��ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�
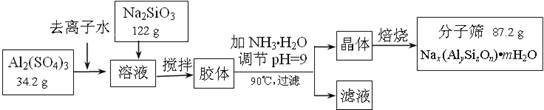
��1���ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)=���� �� ��
��2������������������Һ����Ҫ�ɷ�Ϊ���� �� ��д��ѧʽ����
��3������������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10������ͨ������ȷ���÷���ɸ�Ļ�ѧʽ��д��������̣���
��4������ɸ�Ŀ�ֱ��Ϊ4Å��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч���������飨����ֱ��Ϊ4.65Å�����춡�飨����ֱ��Ϊ5.6Å��Ӧ��ѡ������ �� �͵ķ���ɸ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)����ɸ���������ʵķ����ᴿ��ij���ͺŵķ���ɸ�Ĺ�ҵ�������̿ɼ�ʾ���£�
![]()
��1���ڼ�NH3��H2O����pH�Ĺ����У���pH���Ʋ�������Al(OH)3���ɡ���֪�����£�Al(OH)3��Һ��pH=3����Ksp=1��10-36��������c(Al3+)=���� �� ��
��2������������������Һ����Ҫ�ɷ�Ϊ���� �� ��д��ѧʽ����
��3������������������Ԫ�غ�Ԫ�ؾ�û����ģ���ԭ�ӵ�������Ϊ10������ͨ������ȷ���÷���ɸ�Ļ�ѧʽ��д��������̣���
��4������ɸ�Ŀ�ֱ��Ϊ4Å��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�3A�ͷ���ɸ��Ҫ��Ч���������飨����ֱ��Ϊ4.65Å�����춡�飨����ֱ��Ϊ5.6Å��Ӧ��ѡ�������� �͵ķ���ɸ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com