£Ø2011?½É½ŹŠÄ£Äā£©ŅŃÖŖĻĀĶ¼ĆæŅ»·½æņÖŠµÄ×ÖÄø“ś±ķŅ»ÖÖ·“Ó¦Īļ»ņÉś³ÉĪļ£ØĶ¼ÖŠ²æ·ÖÉś³ÉĪļƻӊĮŠ³ö£©£®»ÆŗĻĪļAŹĒÓÉ1828ÄźµĀ¹ś»Æѧ¼ŅĪ¬ĄÕĶعżÕō·¢ĒčĖįļ§µĆµ½µÄÓŠ»śĪļ£¬ĪŖ“Ė£¬Ėū³¹µ×“ņĘĘĮĖÓŠ»ś»ÆŗĻĪļÖ»ÄÜÓÉÉśĪļµÄĻø°ūÖŠĢįČ”µÄ”°ÉśĆüĮ¦”±ĀŪ£®AµÄ»ÆѧŹ½æɱķŹ¾ĪŖXY
4ZM
2£¬×é³ÉAµÄĖÄÖÖŌŖĖŲ¶¼ŹĒ¶ĢÖÜĘŚŌŖĖŲ£¬ĘäŌ×ÓŠņŹżÖ®ŗĶĪŖ22£¬X”¢M”¢Z·Ö±šĪ»ÓŚĻąĮŚµÄÖ÷×壬Ō×ÓŠņŹżŅĄ“ĪŌö“ó£®C”¢D”¢G”¢I”¢J”¢KĪŖĘųĢ壬ĘäÖŠC”¢KµÄĪĀŹŅŠ§Ó¦¾łĻŌÖų£¬KŹĒŗ¬ĒāĮæ×īøßµÄÓŠ»śĪļ£¬DÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£®BĪŖŅ»ÖÖ°×É«¹ĢĢ壬Ęä×ī¼ņŹ½æɱķŹ¾ĪŖXY
2M
2£¬EµÄ»ÆѧŹ½æɱķŹ¾ĪŖX
3M
4£®Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£®
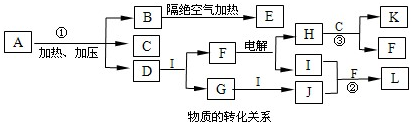
£Ø1£©AŅ²ŹĒŅ»ÖÖ³£ÓƵķŹĮĻ£¬ĘäĆū³ĘŹĒ
ÄņĖŲ»ņĢ¼õ£¶ž°·»ņėå»ņĢ¼õ£°·
ÄņĖŲ»ņĢ¼õ£¶ž°·»ņėå»ņĢ¼õ£°·
£¬ÄæĒ°¹¤ŅµÉĻÓÉ
CO2
CO2
ŗĶ
NH3
NH3
ŌŚŅ»¶ØĢõ¼ž¶ųÖĘµĆ£®
£Ø2£©·“Ó¦¢Ł¢Ś¢ŪµÄ»Æѧ·½³ĢŹ½·Ö±šĪŖ
6£ØNH2£©2CO”śC3H6N6+NH3”ü+3CO2”ü
6£ØNH2£©2CO”śC3H6N6+NH3”ü+3CO2”ü
Ӣ
4NO2+O2+2H2O=4HNO3
4NO2+O2+2H2O=4HNO3
Ӣ
CO2+4H2”śCH4+2H2
CO2+4H2”śCH4+2H2
£®
£Ø3£©I”¢BÖŠMµÄÖŹĮæ·ÖŹżĪŖ
66.7%
66.7%
£»ČōBŹĒŅ»øöŗ¬ÓŠČž¼üµÄ·Ö×Ó£¬“Ó½į¹¹ÉĻæÉæ“×÷ŹĒČż·Ö×Ó£ØXY
2M
2£©¾ŪŗĻ¶ų³É£¬ŹĒŅ»ÖÖµĶ¶¾µÄ»Æ¹¤ŌĮĻ£¬Ä³Š©²»·Ø·Ö×ÓĶłÄĢ·ŪĄļĢķ¼ÓB¶ųÖĘŌģĮĖĪÅĆūÖŠĶāµÄ”°Ó¤Ó׶łÄĢ·ŪŹĀ¼ž”±£¬¾Ż“Ė£¬ĒėÄć»³öBµÄ½į¹¹¼ņŹ½
£®
II”¢ŅŃÖŖ°±·Ö×ӵļü½ĒĪŖ107”ć18”䣬ĻĀĮŠ¹ŲÓŚBµÄĖµ·Ø²»ÕżČ·µÄŹĒ
¢Ü¢ß¢ā
¢Ü¢ß¢ā
£®£ØĢīŠņŗÅ£©
¢Łŗ¬ÓŠ²»±„ŗĶĢ¼Ō×Ó£¬ŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢Éś¼Ó³É·“Ó¦
¢ŚÄÜÓėŃĪĖį·“Ӧɜ³ÉŃĪ
¢ŪŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢ÉśŃõ»Æ·“Ó¦
¢Ü·Ö×ÓÖŠĖłÓŠŌ×Ó¶¼ŌŚĶ¬Ņ»Ę½ĆęÉĻ
¢ŻŌŚøßĪĀĻĀ·Ö½ā·Å³öN
2£¬¹ŹæÉÓĆ×÷×čČ¼¼Į
¢ŽŌŚŅ»¶ØĢõ¼žĻĀæÉÓėĒāĘų·¢Éś¼Ó³É·“Ó¦
¢ßŗ¬µŖĮæµĶÓŚAµÄŗ¬µŖĮæ
¢ąĖüŹĒŅ»ÖÖ¼«ŠŌ»ÆŗĻĪļ£¬Ņ×ČÜÓŚ¼×“¼”¢¼×Č©µČÓŠ»śĪļ
¢į“óĮæŹ³ÓĆŗ¬BĪŪČ¾ÄĢ·ŪµÄÓ¤Ó׶ł»į²śÉśÉö½įŹÆµČĆŚÄņĻµĶ³¼²²”
¢āŹĒ°×É«·ŪÄ©£¬ÓŠ“Ģ¼¤ŠŌĘųĪ¶
£Ø4£©»ÆŗĻĪļEŹĒŅ»ÖÖŠĀŠĶĪŽ»ś²ÄĮĻ£¬ĖüµÄŅ»ÖÖ½į¹¹£Ø¦Ā-X
3M
4£©¾ßÓŠæÉÓė½šøÕŹÆĻąęĒĆĄµÄÓ²¶Č£®ĒėĶĘ²āøĆ²ÄĮĻæÉÄܵÄÓĆĶ¾Ö®Ņ»ŹĒ
×öÄĶÄ„²ÄĮĻµČ
×öÄĶÄ„²ÄĮĻµČ
£®
£Ø5£©ŅŗĢ¬DĄąĖĘH
2O£¬Ņ²ÄÜĪ¢ČõµēĄėĒŅ²śÉśµē×ÓŹżĻąĶ¬µÄĮ½ÖÖĄė×Ó£¬ŌņŅŗĢ¬DµēĄė·½³ĢŹ½ĪŖ
2NH
3
NH
4++NH
2-2NH
3
NH
4++NH
2-£®
£Ø6£©¢ŁŌŚ½į¹¹ÉĻN
2H
4ŗĶDµÄ¹ŲĻµÓŠČēH
2O
2ŗĶH
2OµÄ¹ŲĻµ£®N
2H
4ÄÜ·¢ÉśĻĀĮŠ·“Ó¦£ŗ
N
2H
4+H
3O
+ØTN
2H
+5+H
2O N
2H
4+H
2OØTN
2H
5++OH
-N
2H
5++H
2OØTN
2H
62++OH
- N
2H
5++H
2OØTN
2H
4+H
3O
+¾Ż“ĖæÉµĆ³öµÄ½įĀŪŹĒ
C
C
£®
A£®ėĀĖ®½āĻŌĖįŠŌ”””””””””””””””””” B£®ėĀŌŚĖ®ÖŠµēĄė³öH
+Ąė×Ó
C£®ėĀŹĒ¶žŌŖČõ¼ī”””””””””””””””””” D£®ėĀŹĒ¶žŌŖČõĖį
¢Ś·¢Éä”°ÉńÖŪĘßŗÅ”±·É“¬µÄ³¤Õ÷2ŗÅ»š¼żÓĆĮĖėĀ£ØN
2H
4£©×÷Č¼ĮĻ£¬¶žŃõ»ÆµŖ×÷Ńõ»Æ¼Į£¬Į½Õß·“Ӧɜ³ÉµŖĘųŗĶĘųĢ¬Ė®£®ŅŃÖŖ4g N
2H
4£Øg£©ŌŚÉĻŹö·“Ó¦ÖŠ·Å³ö71kJµÄČČĮ棬Š“³öČČ»Æѧ·½³ĢŹ½
N2H4£Øg£©+NO2£Øg£©=3/2N2£Øg£©+2H2O£Øg£©£»”÷H=-568kJ/mol£®
N2H4£Øg£©+NO2£Øg£©=3/2N2£Øg£©+2H2O£Øg£©£»”÷H=-568kJ/mol£®
£®

 Š”ѧæĪŹ±×÷ŅµČ«ĶØĮ·°øĻµĮŠ“š°ø
Š”ѧæĪŹ±×÷ŅµČ«ĶØĮ·°øĻµĮŠ“š°ø ½š°ęæĪĢĆæĪŹ±ŃµĮ·ĻµĮŠ“š°ø
½š°ęæĪĢĆæĪŹ±ŃµĮ·ĻµĮŠ“š°ø µ„ŌŖČ«ÄÜĮ·æ¼¾ķĻµĮŠ“š°ø
µ„ŌŖČ«ÄÜĮ·æ¼¾ķĻµĮŠ“š°ø



 NH4++NH2-
NH4++NH2- NH4++NH2-
NH4++NH2-

