
| n |
| V |


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
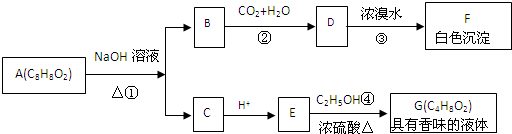
 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�| Ũ���� |
| 170�� |
| ���� |
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g?cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -l30 | 9 | -1l6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
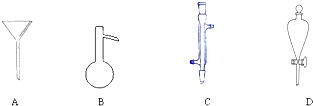
| A������� | B������ |
| C��̼������Һ | D������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
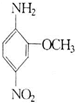 2-����-5�����������׳ƺ�ɫ��B����Ҫ��������ά֯���Ⱦɫ��Ҳ�����ƽ�ơ���졢�ڵ��л����ϣ���ṹ��ʽ��ͼ��ʾ��������ʽ���ɫ��B��ͬ���Ұ�����-NH2����������-NO2��ֱ�����ڱ����ϲ��ʶ�λʱ��ͬ���칹����Ŀ��������ɫ��B������Ϊ��������
2-����-5�����������׳ƺ�ɫ��B����Ҫ��������ά֯���Ⱦɫ��Ҳ�����ƽ�ơ���졢�ڵ��л����ϣ���ṹ��ʽ��ͼ��ʾ��������ʽ���ɫ��B��ͬ���Ұ�����-NH2����������-NO2��ֱ�����ڱ����ϲ��ʶ�λʱ��ͬ���칹����Ŀ��������ɫ��B������Ϊ��������| A��4�� | B��6�� | C��8�� | D��10�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��M+����c��OH-����c��A-����c��H+�� |
| B��c��M+����c��A-����c��H+����c��OH-�� |
| C��c��M+����c��A-����c��OH-����c��H+�� |
| D��c��M+����c��H+����c��A-����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��CH3COO-����c��Na+�� |
| B��c��CH3COOH����c��CH3COO-�� |
| C��2c��H+��=c��CH3COO-��-c��CH3COOH�� |
| D��c��CH3COOH��+c��CH3COO-��=0.02mol/L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com